3 Pathology Chronic Venous and Lympho-Venous Insufficiency Lymphedema is a very common and serious condition, affecting at least 3 million Americans. It occurs if the transport capacity (TC) of the lymphatic system has fallen below the normal lymphatic load (LL; see Chapter 2, Mechanical Insufficiency), resulting in an abnormal accumulation of water and proteins principally in the subcutaneous tissues. Lymphedema may be present in the extremities, trunk, abdomen, head and neck, external genitalia, and internal organs; its onset is gradual in some patients and sudden in others. Most patients in the Western Hemisphere develop lymphedema after surgery and/or radiation therapy for various cancers (breast, uterus, prostate, bladder, lymphoma, melanoma), in which case it is referred to as secondary lymphedema. Other patients develop it without obvious cause at different stages in life (primary lymphedema), and still others develop it after trauma or deep vein thrombosis. In developing countries, parasites (filariasis) account for millions of cases. Lymphedema is serious because of its long-term physical and psychosocial consequences for patients; it continues to progress if left untreated. If lymphedema combines with other pathologies (cardiac and venous insufficiency, chronic arthritic conditions, etc.), the pathophysiological effects are further exacerbated due to the additional stress placed on the already compromised lymphatic system (see Chapter 2, Combined Insufficiency). Its cosmetic deformities are difficult to hide, and complications do occur frequently (fibrosis, cellulitis, lymphangitis, lymphorrhea, etc.). Lymphedema is also serious because of the pervasive lack of medical expertise in the diagnosis and treatment of this condition and the tendency of clinicians to trivialize lymphedema in patients who have been treated for cancer. No specific studies on the incidence of lymphedema have been performed to date, and estimated rates reported in the literature vary widely. Worldwide, 140 million to 250 million cases of lymphedema are estimated to exist, with filariasis, a parasitic infestation (see Secondary Lymphedema later in this chapter), being the most common cause. In the United States, the highest incidence of lymphedema is observed following breast cancer surgery, particularly among those who undergo radiation therapy following axillary lymph node dissection. Other than skin cancer, breast cancer is the most common type of cancer among women in the United States. All women are at risk for developing breast cancer. A woman’s chance of developing breast cancer increases with age. The majority of breast cancer cases occur in women over 50 years of age. Although breast cancer is less common at a young age, younger women tend to have more aggressive types of breast cancer than older women, which may explain why survival rates are lower among younger women. Incidence also varies within ethnic groups and geographical location within the United States (Table 3.1). Table 3.1 Incidence of breast cancer by age
Lymphedema
Definition
Incidence of Lymphedema
By age 30 | 1 out of 2212 |
By age 40 | 1 out of 235 |
By age 50 | 1 out of 54 |
By age 60 | 1 out of 23 |
By age 70 | 1 out of 14 |
By age 80 | 1 out of 10 |
Ever | 1 out of 8 |
Source: National Cancer Institute, 1999
Generally, it can be said that 1 in 8 women in the United States will develop breast cancer during the course of their lives. (More information can be found on the National Institute for Cancer Website at http://cancer.gov/cancerinformation, accessed June 9, 2012.)
Other cancer survivors at risk for lymphedema include those who have undergone surgery and/or radiation treatment for malignant melanoma of the upper or lower extremities; prostate cancer; gynecologic cancers; ovarian, testicular, and prostate cancers; and colorectal, pancreatic, or liver cancers. Some studies report the incidence of lower extremity lymphedema (and/or genital lymphedema) after radical lymph node dissection in prostate cancer to be more than 70%.
It is generally thought that the more lymph nodes are removed during any surgical procedure, the higher the incidence of lymphedema. The true numbers of patients suffering from any form of lymphedema are unknown.
Most statistics are available on the incidence of upper extremity lymphedema following breast cancer surgery in women. Studies show incidences varying from 6–7% of breast cancer patients (a report in the April 1984 issue of Breast Cancer Digest found that ~50–70% of patients who have had axillary node surgery will develop lymphedema). In general, studies with longer follow-up show higher incidence and more severe swelling. Some authors feel that with the more conservative surgical procedure (modified radical mastectomy), the incidence has decreased. Surgeons hope that the sentinel node procedure (see discussion in Secondary Lymphedema later in this chapter) will reduce lymphedema because it removes fewer nodes. Presently, there is not enough follow-up information available to state this with certainty.
Based on the numbers above and other statistics, it is estimated that 2 million to 3 million secondary and 1 million to 2 million primary lymphedema cases are currently in existence in the United States.
Lymphedema may develop anytime during the course of a lifetime in primary cases. Secondary cases may occur immediately postoperative, within a few months, a couple of years, or 20 years or more after surgery.
Lymphedema Incidence among Non-Breast Cancer Patients
Lymphedema is recognized as a significant breast cancer survivorship issue; however, this chronic, progressive condition can also occur after the treatment of other solid tumors particularly those requiring lymph node dissections. While there have been several studies examining the incidence and risk factors for lymphedema following the treatment of breast cancer,1 less is known about lymphedema following the treatment of other tumors.
Our research group performed a systematic review and meta-analysis of the oncology-related medical literature to determine the reported incidence of and risk factors for lymphedema after treatment of cancers other than breast carcinoma.2 We searched three major medical indices (MEDLINE, Cochrane Library databases, and Scopus) to identify all prospective studies of post-treatment lymphedema published from 1972 to 2010. These studies were identified and categorized according to the type of malignancy. Detailed information related to the surgical procedure, radiation therapy, follow-up interval, lymphedema measurement criteria, and lymphedema incidence were extracted from each article. The Quality Assessment Tool for Diagnostic Accuracy Studies3 was used to score individual study quality, with scores ranging from 0 (worst quality) to 14 (best quality). Overall estimates of lymphedema incidence were calculated using weighted averages, on the basis of study size, for each type of malignancy.
Forty-seven eligible studies were identified that evaluated secondary lymphedema in patients with melanoma (n = 19 studies), gynecologic cancer (n = 25), genitourinary cancer (n = 8), head and neck cancer (n = 1), and sarcoma (n = 1). The median (range) study quality scores were as follows: melanoma, 7 (4–10); gynecologic cancer, 7 (4–10); genitourinary cancer, 5 (3–9); head and neck cancer, 5 (4–10); and sarcoma, 7. Of the 8341 patients included in these reports, 16% had been diagnosed with lymphedema, with a reported incidence of 0%–73%. This variability in the reported incidence can be attributed to the significant heterogeneity among the studies’ clinical lymphedema definitions, lymphedema measurement methods, and follow-up durations.
Patients with sarcoma had the highest pooled incidence of lymphedema (30%), followed by patients with gynecologic cancer (20%), melanoma (16%), genitourinary cancer (10%), and head and neck cancer (4%) (Table 3.2). Among patients with melanoma, those undergoing axillary lymph node dissection had a much lower incidence of lymphedema (5%) than did those undergoing inguino-femoral lymph node dissection (28%). Overall, 2837 patients in 22 studies underwent pelvic lymph node dissection for various malignancies; their incidence of lymphedema was 22%. The incidence of lymphedema among 1716 patients in 18 studies treated with radiation was 31%.
Lymphedema Genetics
Hereditary Lymphedema
In the United States and Europe, the predominant presentation of lymphedema is as a secondary condition following cancer treatment. However, there are also forms of primary lymphedema which run in families (hereditary) and have been recognized as early as 1892 by Milroy.4 Despite this long history, it is only in the last 10–15 years with the explosion in molecular biology and specifically molecular lymphology that advances in lympho-vascular genetics have taken place.5 The true incidence is not clear because standardized evaluation protocols do not exist, few centers are equipped to focus on patients with genelinked lymphatic abnormalities, and testing options are limited. In patients with primary lymphedema, based on available evidence including testing, it is reasonable to estimate that 5%–10% are hereditary. There is a slightly higher incidence in females with males generally presenting at birth and females at puberty; however, this feature is not of diagnostic value. Examination of the lymphatic system abnormalities in these patients has demonstrated developmental aplasia, hypoplasia, and hyperplasia of the lymphatic vessels and nodes as the underlying cause of the lymphedema. The majority of patients described so far have autosomal dominant mutations meaning that a single mutated gene passed on to the next generation is sufficient to cause disease. Others are autosomal recessive requiring a mutated gene from each parent. Some of these genes have reduced penetrance meaning that even though the gene may be present, its effect may not be evident. There are also genes that have varied levels and types of presentation. These features understandably require careful evaluation by the clinical team.
The Online Mendelian Inheritance in Man (OMIM) catalog focuses mainly on inherited genetic diseases.6 This catalog is frequently updated and easily searchable. Focusing on lymphedema or lymphangiectasia, there are almost 40 syndromes that have lymphedema as a component, and many of these have multiple phenotypic abnormalities of the lymphatic system and a variety of other systems involved.6 There are also additional syndromes that do not yet have entries in OMIM.7
Known Genes
There are seven genes implicated so far in syndromes with either lymphedema as a primary phenotype or as a consistent feature, two genes without a specifically defined associated lymphedema syndrome, and six syndromes with chromosomal abnormalities (Table 3.3). These mutations and chromosomal abnormalities are spread across the entire human genome, and it appears unlikely that genes yet to be discovered will be segregated to more focused areas. It is interesting to note that all the mutations so far have been found on the q or long arm of the chromosomes. Whether this will also be the case for new genes remains to be seen.
Approach to Clinical Evaluation
Although major advances in understanding the role of specific genes in development of the lymphatic system and lymphedema-related syndromes have occurred recently, defining the mechanistic link between the clinical phenotype and the genotype remains incomplete. The first step in approaching the patient is detailed evaluation by the clinical team of the history and physical examination, which remains paramount in phenotyping the patient. This phenotyping should also include imaging of the lymphatic system to define more precisely the anatomical and functional defects. Without clear distinctions between patients with specifically defined syndromes, the sophisticated genetic techniques will not be as powerful.
The second step is to determine the genotype. This task will largely fall to the geneticist or knowledgeable physician on the team. After obtaining consent from the patient, a requirement due to the type of information which can be revealed by analysis of genetic material regardless of whether the patient is in a study or not, a sample is collected to isolate DNA. This sample can be blood, scrapings of cheek cells, mouthwash following vigorous rinsing, or a small piece of tissue obtained from an operative procedure. Standardized protocols are used to isolate DNA from these samples. The number of family members who are available and whether the search is for a specific known gene(s) or for a new gene will determine what type of analysis is employed. Sophisticated linkage analysis may take place, whole genome, specific gene, or exome sequencing may be utilized, or newer gene-chip technology could be used. Different clinical centers have varying levels of expertise with these techniques and some specific gene testing is commercially available. Associated costs should be considered.
The Future
From studies in mice it is known that there are many more genes that impact lymphatic system development and function which do not yet have a corresponding gene in man, or the corresponding mutations have yet to be discovered. There are also many human syndromes, which so far have no known associated gene defect. These areas are clearly where future advances will be made. In addition, improvements in availability and sophistication of genetic testing, more comprehensive and careful phenotyping of the physical manifestations, and diagnostic imaging of the lymphatic system by clinicians should move the field forward.
The goal of investigating the genetics of lymphedema-related syndromes is to translate these basic science discoveries back into the clinic and impact lives of patients. Genes crucial for the growth and development of the lymphatic system can be identified, then this knowledge can be harnessed to stimulate (or inhibit) lymphatic system growth and function when and where it is defective or dysfunctional. In addition, by carefully defining the syndromes that manifest lymphedema, specific genetic treatment plans can be devised including pinpointing of the optimal timing to correct or ameliorate the defects. Unfortunately, the recent history of gene therapy has yet to yield the anticipated success and practical applications and our understanding of the specific genes, expressions, interactions, and context for future treatment remains limited.
Etiology of Lymphedema
Lymphedema can be classified as primary or secondary, based on underlying etiology. However, this classification usually has little significance in determining the method of treatment (Table 3.4).
Table 3.4 Etiology of lymphedema
Primary lymphedema | Secondary lymphedema |
• Aplasia • Hypoplasia • Hyperplasia (lymphangiectasia/megalymphatics) • Fibrosis of lymph nodes • Agenesis of lymph nodes • Congenital • < 35 years of age: lymphedema praecox • > 35 years of age: lymphedema tarda | • Dissection of lymph nodes • Radiation • Trauma • Surgery • Infection • Malignancies • Chronic venous insufficiency • Immobility • Self-induced |
Fig. 3.1 Reduced transport capacity in the subclinical stage of lymphedema. LL, lymphatic loads or lymph volume; LTV, lymph time volume (TC = LTVmax); TC, transport capacity of the lymphatic system.
Primary Lymphedema
Primary lymphedema represents a developmental abnormality (dysplasia) of the lymphatic system, which is either congenital or hereditary. It can present as a variety of abnormalities.
Hypoplasia. This most common form of dysplasia refers to the incomplete development of lymph vessels; that is, the number of lymph collectors is reduced, and the diameter of existing lymph vessels is smaller than normal.
Hyperplasia. The diameter of lymph collectors is larger than normal in this dysplasia (lymphangiectasia or megalymphatics). The dilation of the lymph collectors results in a malfunction of the valvular system within the collectors, which often leads to lymphatic reflux.
Aplasia. The absence of single lymph collectors, capillaries, or lymph nodes associated with this abnormality may be a cause for the development of primary lymphedema.
Fibrosis of the inguinal lymph nodes (Kinmonth syndrome) presents an additional cause for the onset of primary lymphedema. The fibrotic changes primarily affect the capsular and trabecular area of the involved lymph nodes. This may affect lymph transport in the afferent lymph collectors.
With the understanding of basic lymphatic system physiology, it becomes evident that the transport capacity of the lymphatic system in all the abnormalities listed above is reduced (Fig. 3.1). As discussed in Chapter 2, lymphedema occurs if the transport capacity of the lymphatic system falls below the normal lymphatic load.
Although the developmental abnormalities are present at birth, lymphedema may develop at some point later in life. It may not develop at all as long as the (reduced) transport capacity of the lymphatic system is sufficient to manage the lymphatic loads. Primary lymphedema is often classified by the age of the patient at the onset of swelling.
Congenital lymphedema is clinically evident at birth or within the first 2 years of life. A subgroup of patients with congenital lymphedema has a familial pattern of inheritance, which is termed Milroy’s disease. If primary lymphedema presents after birth but before the age of 35, it is called lymphedema praecox, which is the most common form of primary lymphedema and most often arises during puberty or pregnancy. Lymphedema tarda is relatively rare and develops after the age of 35.
Primary lymphedema almost exclusively affects the lower extremity (unilateral and bi-lateral) and involves mostly females. The swelling usually starts at the foot and ankle and gradually involves the remainder of the extremity. It may occur without any known impetus or may develop after minor trauma (insect bites, injections, sprains, strains, burns, cuts), infections, or immobility. These triggering factors produce additional stress to the already impaired lymphatic system, resulting in mechanical insufficiency (Fig. 3.2).
Secondary Lymphedema
The mechanical insufficiency present in secondary lymphedema is caused by a known insult to the lymphatic system.
Most common causes for secondary lymphedema include surgery and radiation, trauma, infection, malignant tumors, immobility, and chronic venous insufficiency.
Lymphedema may also be self-induced.
Surgery and radiation: As outlined earlier, this is by far the most common cause for secondary lymphedema in the United States. Surgical procedures in cancer therapy commonly include the removal (dissection) of lymph nodes. The goal of these procedures is to eliminate the cancer cells and to save the patient’s life.
A side effect in lymph node dissection is the disruption in the lymph transport. If the remaining lymphatics are unable to manage the lymphatic load, secondary lymphedema will develop.
In the early years of breast cancer surgery radical mastectomy was the only option available for patients. Radical mastectomy includes the removal of the entire mammary gland, the axillary lymph nodes, and the pectoralis muscles under the breast. Although common in the past, radical mastectomy is now rarely performed and is recommended only if the cancer cells have spread to the muscles under the mammary gland. Modified radical mastectomy is now more commonly performed. This procedure includes the removal of the breast and part of the axillary lymph nodes. In certain forms of breast cancer, a simple or total mastectomy is performed, in which only the mammary gland, but not the axillary lymph nodes, is removed.
Today, many women with breast cancer are given the choice between mastectomy and lumpectomy. In lumpectomy, also referred to as breast-conserving surgery, only the part of the mammary gland containing the malignant tumor and some of the normal surrounding tissue are removed. Most women after breast surgery, especially after lumpectomy, receive radiation treatment (Fig. 3.3).
Sentinel lymph node biopsy, a relatively new technique, was developed to determine if cancer cells have spread to the axillary nodes and trunks, without having to perform a traditional axillary lymph node dissection during which, on average, ~10–15 axillary lymph nodes are removed. A sentinel lymph node biopsy requires the removal of only those lymph nodes to which the mammary gland drains first (sentinel nodes) before reaching the rest of the axillary lymph nodes. A pathologist then closely reviews one to three lymph nodes. If they do not contain malignant cells, the chance that the remaining axillary nodes are cancer free is ~ 95%, and removal of additional axillary nodes may be avoided.
Radiation (or radiotherapy) is the treatment of cancer and other diseases with ionizing radiation. The goal of this therapy is to destroy cancer cells that may linger after surgery. It uses either precisely aimed, high-energy, external beam radiation or radioactive seeds that are implanted in the tumor area. Malignant cells often grow at a faster rate than normal cells; this makes many cancers very sensitive; that is, vulnerable to radiation.
Although radiation damages both cancer cells and normal cells, the normal cells are able to repair themselves and continue to function properly. Radiation is usually administered 5 days a week for several weeks and may also contribute to the onset of lymphedema. Rays can cause fibrosis in the tissues, leading to an impaired lymph transport, and hinder the regeneration of lymph vessels. Radiation may also affect nervous tissue, which can result in numerous problems affecting either the lymphedema itself or the patient’s ability and compliance during the treatment of the lymphedema (radiation plexopathy; see Complications in Lymphedema later in this chapter).
Trauma: Traumatic insults involving the lymphatic system may cause a significant reduction in lymph flow, resulting in secondary lymphedema (burns, larger skin abrasions). Scar tissue hinders regeneration (lympholymphatic anastomosis) of lymph collectors, further exacerbating the problem. Post-traumatic secondary lymphedema develops from a mechanical insufficiency of the lymphatic system as a result of tissue lesions and should not be confused with post-traumatic edema. Post-traumatic edema is a local result due to trauma, which usually recedes after a few days (see Lipedema later in this chapter).
Fig. 3.3 a, b a. Secondary lymphedema of the left upper extremity. b. Secondary lymphedema of the left upper extremity following bilateral mastectomy.
Infection: Recurrent acute or chronic inflammatory processes affecting the lymphatic system may result in mechanical insufficiency. If inflammation involves lymph nodes (lymphadenitis) or collectors (lymphangitis), the walls tend to become fibrotic and the lymph fluid coagulates and obliterates the lymphatics, thus creating blockage to the lymph flow. In addition to the existing mechanical insufficiency, there is an increase in the volume of lymphatic load, resulting in combined insufficiency.
Lymph node and lymph vessel infections can be caused by bacteria (especially Streptococcus) and fungal infections. The inflammatory process affecting intra- and periarticular tissues in rheumatoid arthritis may spread to the lymphatics, presenting another cause for mechanical insufficiency (see Traumatic Edema later in this chapter).
The most common cause for inflammation of the lymphatic system and lymphedema in general is filariasis.
Lymphatic Filariasis
Lymphatic filariasis is the primary cause of lymphedema worldwide and is a painful and extremely disfiguring disease, which has been identified by the World Health Organization (WHO) as a leading cause for permanent and long-term disability in the world. It is a tropical disease, endemic in more than 80 countries in Africa, India, Southeast Asia, and South America, as well as in the Pacific islands and the Caribbean. Lymphatic filariasis is rare in the United States, and it is likely that those who contract it will have visited endemic regions.
According to the WHO, 1.3 billion individuals are at risk from the disease and over 120 million people are currently affected, with ~40 million being disfigured by lymphedema and suffering from recurrent infections and other secondary conditions.
Filariasis is caused by three types of round parasitic filarial worms with Wuchereria bancrofti being the most common type. The other types, Brugia malayi and Brugia timori are endemic to Southeast Asia.
Lymphatic filariasis is transmitted to humans by different types of mosquitos that bite while carrying infective-stage larvae. At the time of the bite, the larvae enter the wound and are deposited in the victim’s skin; from there the parasitic larvae migrate to the lymphatic system, where over a period of 6–12 months they develop into adult worms and mate. Male and female worms live together and form “nests” in the nodes and vessels of the lymphatic system. Adult worms live for a period of ~4–6 years; male worms can grow to 3–4 cm in length, whereas females can reach 8–10 cm.
The females produce millions of microscopic worms (microfilariae) during their lifetime, which circulate in the host’s bloodstream and are then again ingested by biting mosquitos. Once inside the mosquito, the microfilariae develop into infective-stage larvae, which then again are transmitted to other individuals, thus completing the transmission cycle (Fig. 3.4a).
During their lifetime inside the host’s lymphatic system, the worms cause dilation of and damage to the lymphatics, restricting the normal flow of lymph, and cause swelling, fibrosis, and infections to lymph vessels and nodes (lymphangitis, lymphadenitis).
While infection by larvae generally occurs in childhood in individuals living in endemic countries, the painful and disfiguring symptoms of this condition typically manifest themselves later in life.

Fig. 3.4 a, b
a Lifecycle of Wuchereria bancrofti. (Reprinted with permission from the Centers for Disease Control and Prevention.)
b A Nigerian man with elephantiasis, the most extreme form of lymphatic filariasis. (Reprinted with permission from The Carter Center/Emily Staub.)
Lymphatic filariasis may present asymptomatically with no external signs of disfigurement or infection but with acute (infections, fever, swelling) or chronic subclinical lymphatic damage. The chronic stage includes lymphedema, which can grow to monstrous proportions and may affect the extremities (most often the legs), breasts, and the external genitalia (labia, scrotum, and penis) causing pain, disability, and sexual dysfunction (Fig. 3.4b).
Lymphatic filariasis is typically diagnosed through blood tests that detect the presence of microfilariae in the blood, and antigen detection tests (ICT).
The primary treatment approach for individuals affected by lymphatic filariasis is pharmaceutical (diethylcarbamazine [DEC], albendazole, and ivermectin) and aims to eliminate adult worms and circulating microfilariae, thus interrupting the transmission cycle. Another important goal is to eliminate lymphatic filariasis as a public health problem by preventive measures involving mass drug administration covering the entire at-risk population of a country. The goal of the Global Alliance to Eliminate Lymphatic Filariasis (GAELF) is to stop the spread of filarial infection and to eradicate this disease through distribution of free medication. To interrupt the transmission of infection, mass drug administration should be implemented in endemic regions for a period of 4–6 years.
Foreigners visiting endemic countries are rarely infected; however, as a preventive measure mosquito bites should be avoided by sleeping under a mosquito net, using insect repellents, wearing long-sleeved shirts and long pants and refraining from being outside between dusk and dawn, when mosquitos are most active.
Lymphedema caused by lymphatic filariasis can be treated effectively with complete decongestive therapy (CDT), if available. Other measures to improve lymphedema and infections are patient education in self-care including hygiene, skin care, compression therapy, exercises and elevation of the affected extremity.
Malignant tumors: Malignant tumors may mechanically block the lymph flow by pressing against lymphatic structures from the outside (see Complications in Lymphedema later in this chapter). Malignant cells may also infiltrate the lymphatic system and proliferate in either lymph vessels (malignant lymphangiosis) or lymph nodes, thus blocking the flow of lymph (Fig. 3.5). Modified CDT protocols may be applied to address the symptoms associated with malignancies (Fig. 3.6).
Chronic venous insufficiency: Insufficient venous return results in an increase in venous blood pressure. The subsequent elevation in blood capillary pressure causes an increase in net filtrate (see Chronic Venous and Lympho-venous Insufficiency later in this chapter). In its primary function to actively prevent edema, the lymphatic system tries to compensate for the higher volume of the lymphatic load of water by the activation of its safety factor (see Chapter 2, Safety Factor of the Lymphatic System). Without initiation of adequate therapy for the venous problem, the lymphatic system may develop a mechanical insufficiency (combined insufficiency) over time, due to the constant strain (Fig. 3.7).
Immobility: If left without proper care, immobility caused by injuries to the spinal cord, stroke, or cerebral hemorrhage may eventually result in similar problems as discussed earlier (e.g., insufficient venous return with subsequent lymphatic overload).
Self-induced lymphedema: By use of a tourniquet (bandages, rubber band), some individuals produce a combination of venous and lymphatic obstruction on an extremity that produces the signs and symptoms of lymphedema. The ligature mark is usually easily identifiable just proximal to the swelling. This condition is extremely rare. If a therapist suspects self-induced lymphedema (also known as artificial lymphedema), it is recommended that he or she contact the referring physician following the evaluation (Fig. 3.8).
Table 3.5 Stages of lymphedema with typical symptoms
Stages of lymphedema | Characteristics |
• Latency stage | • No swelling |
• Lymphangiopathy (also stage 0/prestage/subclinical stage) | • Reduced transport capacity (TC) • “Normal” tissue consistency |
Stage 1 (reversible stage) | • Edema is soft (pitting) • No secondary tissue changes • Elevation reduces swelling |
Stage 2 (spontaneously irreversible stage) | • Lymphostatic fibrosis • Hardening of the tissue (no pitting) • Stemmer sign positive • Frequent infections |
Stage 3 (lymphostatic elephantiasis) | • Extreme increase in volume and tissue texture with typical skin changes (papillomas, deep skinfolds, etc.) • Stemmer sign positive |
Fig. 3.8 Self-induced lymphedema of the left lower extremity (note the ligature mark on the left knee).
Stages of Lymphedema
Currently, there is no cure or permanent remedy for lymphedema. The transport capacity in the damaged lymph vessels cannot be restored to its original level (see Chapter 2, Fig. 2.13).
If lymphedema is present, the lymphatic system is mechanically insufficient; that is, the transport capacity has fallen below the normal lymphatic load.
Although the swelling may recede somewhat during the night in some early-stage cases, lymphedema is a progressive condition. Regardless of genesis, lymphedema, in most cases, will gradually progress through its stages, if left untreated (Table 3.5).
There is no specific period of time for a patient to remain in a particular stage. For example, a patient will not necessarily be in stage 1 for 4 months and then progress to stage 2 for 6 months before moving to stage 3.
Stage 0
This stage is also known as the subclinical stage, or prestage, of lymphedema. In this stage, the transport capacity of the lymphatic system is subnormal, yet remains sufficient to manage the (normal) lymphatic load (see Fig. 3.1). This situation results in a limited functional reserve (FR) of the lymphatic system (see Chapter 2, Lymph Time Volume and Transport Capacity of the Lymphatic System).
Patients who have undergone surgery (or had trauma) involving the lymphatic system and do not experience the onset of lymphedema are said to be in a latency stage, which is a subcategory of stage 0. For example, those women who have had surgery for breast cancer (with or without lymph node dissection and radiation) and do not present with postmastectomy/lumpectomy lymphedema are considered to be in a latency stage. Again, in these cases, the TC is subnormal but still sufficient to drain the normal LL.
A condition known as lymphangiopathy presents if the TC is reduced by congenital malformations (dysplasia) of the lymphatic system. As long as the subnormal TC can manage the LL, lymphedema is not clinically present.
Patients in a prestage are “at risk” of developing lymphedema. The reduction in functional reserve results in a fragile balance between the subnormal TC and the LL. The onset of lymphedema correlates to the ability of the lymphatic system to compensate for any added stress to the system or the frequency of certain occurrences that may cause an increase in lymphatic load of water (or water and protein) in the limb at risk.
Patient information and education, especially following surgical procedures, can dramatically reduce the risk of developing lymphedema (see Chapter 5, Precautions and Lymphedema and Air Travel).
Stage 1
This stage, also known as the reversible stage, is characterized by soft-tissue pliability without any fibrotic alterations. Pitting is easily induced, and the swelling retains the indentation produced by the (thumb) pressure for some time (Fig. 3.9). In early stage 1, it is possible for the swelling to recede overnight.
With proper management in this early stage, it is possible for the patient to expect reduction of the extremity to a normal size (compared with the uninvolved limb). Without proper care, progression into stage 2 in the vast majority of cases is inevitable.
It is difficult to distinguish stage 1 lymphedema from edemas of other geneses. The clinician needs to take into account the patient’s history and whether the swelling resolves with conventional management (compression, elevation) or not (refer to Diagnosis and Evaluation of Lymphedema later in this chapter).
Stage 2
Stage 2, also known as spontaneously irreversible lymphedema, is primarily identified by tissue proliferation and subsequent fibrosis (lymphostatic fibrosis). Over time the tissue becomes more indurated, and pitting is difficult to induce. In stage 2, the Stemmer sign is positive (Fig. 3.10). A Stemmer sign is positive if the skin from the dorsum of the fingers and toes cannot be lifted, or lifted only with difficulty (compared with the uninvolved side). A positive Stemmer sign is considered accurate to diagnose lymphedema of the extremities; the absence of a Stemmer sign, however, does not exclude the presence of lymphedema (false-negative Stemmer sign).
In many cases, the volume of the swelling increases, which exacerbates the already compromised local immune defense (increased diffusion distance). Because of this, infections (cellulitis) in this stage are common.
Volume reduction can be expected if proper treatment is initiated in this stage of lymphedema. In most cases, the indurated tissue will not completely recede in the intensive phase of complete decongestive therapy (see Chapter 4). Reduction of fibrotic tissue is achieved mainly in the second phase of CDT with compression and good patient compliance (Fig. 3.11).
Fig. 3.11 Primary lymphedema of the right lower extremity before (left) and after (right) complete de-congestive therapy.
Lymphedema often stabilizes in stage 2. In those patients suffering from recurrent infections, the lymphedema may develop into stage 3, lymphostatic elephantiasis.
Stage 3 (Lymphostatic Elephantiasis)
Typical for this stage are an increase in volume of the lymphostatic edema and further progression of the tissue changes. Lymphostatic fibrosis increases in firmness, and other skin alterations, such as papillomas, cysts, and fistulas, hyperkeratosis, mycotic infections of the nails and skin, and ulcerations, develop frequently. Pitting may or may not be present. The natural skinfolds, especially on the dorsum of the wrist and ankle, deepen, and the Stemmer sign becomes more prominent. In many cases, cellulitis is recurrent (Fig. 3.12).
If lymphedema management starts in this stage, reduction can still be expected. To achieve good results, it is necessary to extend the duration of the intensive phase of complete decongestive therapy (CDT). In many cases, the intensive phase has to be repeated several times. Even extreme cases of lymphostatic elephantiasis can be reduced to a normal or near normal size with proper care and patient compliance (see Therapeutic Approach to Lymphedema later in this chapter).
Tissue changes or the progression of fibrosis remains the clinical trait to distinguish between the stages of lymphedema.
Tissue changes commonly seen in the progression of lymphedema are proliferation of connective tissue cells, production of collagen fibers, and an increase in fatty deposits and fibrotic changes (lymphostatic fibrosis). The fibrotic tissue tends to become sclerotic over time, increasing in firmness. Lymphostatic fibrosis is initially noticed at the distal end of the extremities, fingers, and toes (Fig. 3.13).
Pitting is generally more pronounced in the early stages of lymphedema and occurs if pressure is applied with the examiner’s thumb on the edematous tissue. Pitting is usually tested on the distal extremity (preferably over bony prominences) and occurs because of the displacement of fluid in the tissue caused by pressure with the flat thumb. The pitting response (indentation produced by pressure) can remain on the tested area for some time if there are minimal fibrotic skin changes present.
Table 3.6 Grading of lymphedema based on severity
Severity of lymphedema | Volume increase |
Minimal | <20% |
Moderate | 20%–40% |
Severe | >40% |
Angiosarcoma (Stewart-Treves syndrome; see also Complications in Lymphedema later in this chapter) may develop in long-lasting lymphedema, particularly in patients with stage 3 lymphedema. This type of angiosarcoma may develop in primary or secondary lymphedema and is characterized by extensive malignancy; it is highly lethal. Reliable data on the incidence of angiosarcoma in lymphedema are not available at this time.
Grading of Lymphedema Based on Severity
Extremity volume is not considered within the different stages of lymphedema. The severity of unilateral lymphedema in relation to volume can be assessed within each stage as minimal (less than 20% increase), moderate (20%–40% increase), or severe (more than 40%) increase in limb volume (Table 3.6).
Precipitating Factors for Lymphedema
For a patient “at risk,” the possible development of lymphedema depends on many factors (see Avoidance Mechanisms later in this chapter). Some patients are able to effectively compensate for a decrease in transport capacity and functional reserve by the regeneration of lymph vessels, utilizing alternative collateral circulation routes and lympho-venous anastomoses, and increasing the lymph time volume of remaining collectors. These patients may not exhibit signs or symptoms of lymphedema as long as the lymphatic system has found a way to compensate.
As discussed earlier, lymphedema may develop anytime during the course of a lifetime in primary cases. In secondary cases, the swelling may occur immediately postoperative, within a few months or a couple of years, or 20 years or more after surgery.
Based on the pathology and pathophysiology, as well as patient reports, certain triggers that cause the onset of lymphedema can be identified (for a more detailed review of precipitating factors and prevention of lymphedema, refer to Chapter 5, Precautions).
Lymphedema Risk Reduction (Venipuncture and Blood Pressure)
The surgical procedures used for individuals affected by breast cancer may be mastectomy, partial mastectomy, or lumpectomy. Along with the actual breast surgery for cancer, axillary lymph nodes are removed and/or radiated. As a result of axillary lymph node clearance, the normal lymphatic drainage from the extremity is impaired, and some patients experience the onset of lymphedema. Accumulated lymph in the edematous arm provides a rich culture medium for bacteria, which makes lymphedematous tissues extremely susceptible to infections. Simple injuries and puncture wounds can develop into local or generalized infections that may produce further lymphatic destruction and blockage. To reduce the risk of these postoperative complications, most patients are advised to not have blood pressure readings taken on, intravenous infusions in, or blood samples taken from the arm on the operated side.
Very few published data are available to document the exact risk of lymphedema from performing blood pressure readings, blood draws and injections on the affected extremity. Lack of research and normal variations in an individual’s lymphatic system (numbers or sizes of lymph nodes) make it difficult to quantify personal risk from each triggering factor.
While further research is needed, health care professionals are encouraged to minimize the risk of lymphedema by taking blood pressure readings and blood draws from and giving injections to the nonaffected limb whenever possible. In patients with breast cancer on both sides, these procedures should be performed on the leg or the foot. If this is not possible, the procedure should be carried out on the nondominant arm. If one side has had no lymph node removal, the arm on that side should be used, regardless of whether it is the dominant arm. In an emergency, however (such as a car accident), and if an intravenous line must be started, medical professionals must be allowed to do what they need to do to start the intravenous line as soon as possible.
If a port is present, blood draws should be taken directly from there. In patients with “bad” veins, good hydration and some form of heat (heat pads, warm water) help to dilate the veins prior to cannulation.
To avoid the onset of lymphedema, or infections in lymphedema, health care professionals should follow expert consensus regarding best practice to avoid lymphedema, and inform patients with breast cancer about their risk for lymphedema. Until further research is available, the National Lymphedema Network’s Position Statement on risk reduction practices should be used to deliver information to patients.
Not all medical professionals are familiar with the precautions for avoiding lymphedema, so patients have to be particularly watchful advocates for themselves. Lymphedema alert bracelets are available from The National Lymphedema Network. Wearing this bracelet increases the odds of remaining lymphedema-free and at the same time educates the medical community.
Increase in blood capillary pressure: Active hyperemia (vasodilation) that results from a local or systemic application causes an increase in blood flow, which ultimately will increase the lymphatic load of water and stress a compromised lymphatic system (see Chapter 2, Fig. 2.9). Examples of active hyperemia include local hot pack, other thermal modalities (diathermy, electrical stimulation, ultrasound), massage, vigorous exercise, and infection of the limb “at risk.” Hot tubs and saunas, hot weather and high humidity, as well as injuries, are additional triggering factors.
Passive vasodilation as a result of the obstruction of the venous return will also result in increased net filtrate and place additional stress on the compromised lymphatic system. Examples include chronic venous insufficiency, cardiac insufficiency, and immobility, as well as the examples listed under the next category.
Fluctuation in weight gain and fluid volumes: Pregnancy and obesity, excessive weight gains during the menstrual cycle (cyclic idiopathic edema), and certain medications are known to trigger the onset of lymphedema by causing additional stress (lymphatic load) on the compromised lymphatic system.
Injury: Even in subclinical stages of lymphedema, the immune response is reduced as a result of edematous saturation of the tissues on a microscopic level. Any insult to the integrity of the skin may cause infection, thus triggering the onset of lymphedema. Examples include insect bites, pet scratches, injections, intravenous cannulation, blood pressure measurements on the involved extremity, cuts, and abrasions.
Changes in pressure: The change in cabin pressure during an airline flight, coupled with inactivity, may trigger the onset of lymphedema. The reduced cabin pressure may allow more fluid into the tissue spaces. Additional inactivity allows for venous pooling, which will eventually cause an increased pressure at the blood capillary level, thereby increasing filtration and the lymphatic loads (see Chapter 5, Lymphedema and Air Travel).
Avoidance Mechanisms
In an effort to maintain fluid homeostasis, the body has the ability to respond to lymphostasis, which may prevent the manifestation of lymphedema. The following discussion will focus on the body’s compensatory mechanisms.
Safety Factor
Lymph collectors not affected by either blockage (surgery, radiation, trauma) or malformation will increase their contraction frequency and amplitude (lymphangiomotoricity) in an effort to compensate for those collectors affected by mechanical insufficiency. These compensating collectors are located in the same tributary area. The mechanisms involving the lymphatic safety factor are described in Chapter 2, Safety Factor of the Lymphatic System.
Collateral Circulation
Lymph collectors circumnavigating blocked areas may be able to avoid the onset of lymphedema by redirecting lymph fluid into areas with sufficient lymph drainage. Lymph collectors of the lateral upper arm, for example, may drain into the supraclavicular lymph nodes. This may be significant in case of axillary lymph node dissection because part of the lymph fluid may be rerouted around the axillary area into the supraclavicular nodes. The chance to avoid the manifestation of lymphedema, in this case, of the arm, is even greater if the individual’s collectors of the lateral upper arm communicate with those located in the radial forearm territory (long upper arm type; see Chapter 1, Superficial Layer). If this connection exists, lymph fluid from the forearm and upper arm would be able to bypass the blocked axillary area into the supraclavicular nodes.
Interterritorial anastomoses present another possible bypass for lymph fluid. If the normal flow of lymph within a truncal territory is interrupted by lymph node dissection, the inter-territorial anastomoses may prevent the onset of swelling in those quadrants that would normally drain into the dissected lymph node groups. The higher the number of anastomoses, as described in Chapter 1, Interterritorial Anastomoses, the better the chance of avoiding lymphedema.
Lympho-Lymphatic Anastomosis
Severed lymph collectors tend to reconnect after a relatively short time (2–3 weeks). Newly formed lymph vessels reconnect the distal with the proximal lymph vessels’ stump (Fig. 3.14). Scar tissue may prevent lympho-lymphatic anastomoses. Lymph collectors separated due to blunt trauma (no skin breakage) will regenerate more effectively than collectors disconnected by incisional trauma.
Fig. 3.14a–d Reconnection of lymph collectors following blunt trauma. a Severed lymph collectors. b Intralymphatic pressure in the distal lymph collector stump increases. c Newly formed lymph vessels connecting the distal and proximal lymph vessel stumps. d Lympho-lymphatic regeneration.
Lympho-Venous Anastomosis
The distal end of a severed lymph collector may connect with an adjacent vein, creating a natural shunt. Lymph fluid then directly voids into venous blood.
Lymph Vessels in the Adventitia of Blood Vessels
Larger blood vessels have their own nutrient blood vessels (vasa vasorum vessels), which supply the wall of the larger arteries and veins with oxygen and nutrients.
There are also lymph vessels in the adventitia of larger blood vessels (lymph vasa vasorum). With lymphedema present, lymphatic loads may reach these lymph vasa vasorum vessels via tissue channels. Lymph vessels in the adventitia of blood vessels have the ability to increase their activity, thus providing an additional drainage pathway for stagnant lymph fluid.
Macrophages
If protein-rich fluid accumulates in the tissues, monocytes leave the blood capillaries in large numbers. Once in the tissues, they are macrophages (phagocytes) and will digest accumulated protein molecules. The subsequent decrease in tissue protein concentration will result in an increased reabsorption and a decrease in net filtrate, which will help to reduce the lymphatic loads. The digested protein molecules are broken down into amino acids, which do not present a lymphatic load, and are removed by the blood circulatory system.
Complications in Lymphedema
Lymphedema is often combined with other pathologies and conditions, which either aggravate the existing symptoms or present additional complicating factors in the treatment of lymphedema.
The following is a list of the most common complications.
Reflux: Retrograde flow of lymph fluid caused by valvular insufficiency of lymph collectors. Valvular insufficiency is the result of hyperplasia, dilation of collectors due to constant strain or blockage of lymph flow, or organic changes in the walls of lymph collectors (mural insufficiency; see Chapter 2, Mechanical Insufficiency).
If valvular insufficiency is present, lymph fluid is propelled not only to proximal but also to distal (retrograde flow) during the contraction of lymph angions. Reflux presents as blisterlike formations (lymphatic cysts) on the surface of the skin, commonly in the axillary (Fig. 3.15), cubital, genital (see Chapter 5, Fig. 5.25), and popliteal areas. Lymphatic cysts contain lymph fluid, which may be clear or chylous. If chylous, the reflux originates from the intestinal lymph system.
Clinical relevance: Lymphatic cysts may easily break open, presenting an entryway for pathogens, which can cause infection. Burst cysts associated with leaking of lymph fluid (lymphorrhea) are termed lymphatic fistulas.
To avoid damage to cysts and to prevent infection, it is recommended to cover the cyst with sterile gauze during treatment (local antibiotics should be applied around the fistulas), and not manually work on or around the cysts and fistulas. Cysts should be padded with soft foam material (donut or U-shaped padding) to avoid direct contact with the bandage materials while the patient wears the bandages.
Radiation fibrosis: Reaction of the skin to irradiation, leaving visible and/or palpable changes in the skin and subcutaneous tissue. The skin in radiation fibrosis appears reddish brown (Fig. 3.16), and superficial blood vessels may be dilated in the irradiated area (telangiectasia) (Fig. 3.17). The newly formed scar tissue may be soft or hard, and the skin may adhere to the underlying fascia.
Clinical relevance: The tissue changes in radiation fibrosis tend to get worse over time, possibly resulting in compression of venous blood vessels and subsequent dilation of superficial veins.
Radiation fibrosis may cause pain, limitations in range of motion if near a joint, paresthesia, pareses, and paralysis, which can occur even years after the radiation therapy was administered.
The skin in radiation fibrosis may also be more fragile. To avoid mechanical damage to the irradiated skin, CDT presents a local contraindication in radiation fibrosis if there is adhesion to the fascia or if the radiated area is painful. Movements designed to mildly stretch the affected skin area should be incorporated into the exercise program. CDT techniques may be applied with lighter pressures if skin discoloration or telangiectasia and/or dilated superficial veins are present and the skin is pliable.
Infection: Bacterial (especially Streptococcus) and fungal infections are common in patients with lymphedema (especially stages 2 and 3). Clinical symptoms of cellulitis are fever and tenderness; the skin is red with indistinct margins (Fig. 3.18).
Fungal infections may involve the skin and/or nails and most often affect the lower extremities (Fig. 3.19). Nails generally take on a yellow color, split, flake, and grow too thick. Symptoms in fungal infections of the skin include itching, crusting, scaling, and maceration between the toes. The skin may be moist or dry and may show a grayish-white film. A sweet odor is often associated with fungal infections.
Clinical relevance: Episodes of cellulitis (or erysipelas) usually require a course of systemic antibiotics. CDT is generally contraindicated until the infection has cleared. Therapy for the fungal infection with local or systemic antifungal medication precedes lymphedema treatment.
Hyperkeratosis: Hypertrophy of the corneous layer of the skin. This condition is often associated with lymphedema, especially on the lower extremity. Wartlike papillomas are generally observed on the feet and toes. Skinfolds may be deepened.
Clinical relevance: Good skin hygiene is necessary to avoid possible infections in the moist skinfolds. Hyperkeratosis may be treated with over-the-counter medication or, in extreme cases, may be surgically removed (after decongestion), especially if papillomas interfere with the donning of compression garments. Because papillomas are elevated nodules, care must be taken to not tear the papillomas while donning the garments.
Scars: Scars located perpendicular to lymph collectors may present a blockage to lymph drainage, especially if the scar tissue adheres to the underlying tissues and/or exceeds 3 mm in width.
Clinical relevance: The treatment of fresh scars is covered later in this chapter under Traumatic Edema. Older scars causing discomfort, blocking lymph flow, or hindering exercise protocols may be treated with techniques and materials designed to soften the indurated tissue (foam, manual techniques, nonthermal ultrasound).
Malignancies: Blockage of the lymphatic return may be caused by malignant tumors, in which case the swelling would be categorized as malignant lymphedema. As described previously in this chapter, a rare form of malignancy may develop as a result of long-standing lymphedema (angiosarcoma or Stewart-Treves syndrome). Malignant cells may also infiltrate the lymphatic system, causing blockage to the lymph flow (malignant lymphangiosis) (Fig. 3.20).
Signs and symptoms of malignancies include sudden onset and fast progression of the swelling, pain (especially in the swollen extremity), paresthesia, paresis and paralysis, enlarged lymph nodes, ulcerations on the skin, varicose veins on the thorax, and elevated shoulder due to pain on the involved side in upper extremity involvement (Fig. 3.21).
Changes in the color and integrity of the skin may also indicate malignancies: cellulitis-like redness is often associated with malignant lymphangiosis (Fig. 3.20) (the redness does not appear suddenly as in cellulitis but develops slowly over the course of weeks and months). Hematoma-like discolorations on the skin may indicate the presence of angiosarcoma.
Clinical relevance: If any of the above symptoms are present, if lymphedema is therapy-resistant, or if there is a sudden relapse in swelling in previously treated lymphedema, a physician must be consulted immediately. Modified CDT protocols may be applied to reduce and alleviate the symptoms associated with malignancies (palliative care).
Paresis and paralysis: Partial or complete loss of motor function may be caused by injuries to peripheral nerves, the spinal cord, stroke or cerebral hemorrhage, infiltration of nervous tissue by malignant tumors, or radiation (radiation plexopathy).
Genital swelling: Frequently present in combination with lower extremity lymphedema. In 40%–60% of the male lymphedema population, the external genitalia (scrotum and/or penis) may also present with significant swelling, in addition to the lower extremity involvement. Females are affected less frequently.
Clinical relevance: These patients should be thoroughly instructed in self-management issues (e.g., hygiene, the application of bandages or pads to the swollen area, appropriate clothing, etc.). It is important that compression garments incorporate the genital swelling (pantyhose, compressive body parts; refer to the appropriate sections in Chapters 4 and 5).
Other pathologies combined with lymphedema: Lymphedema is often combined with other conditions and pathologies that may worsen the symptoms associated with lymphedema or complicate the treatment protocol with additional obstacles.
Clinical relevance: It is necessary to incorporate modifications in the treatment protocol for lymphedema to appropriately address signs and symptoms associated with any additional pathologies.
Examples
Lymphedema and cardiac insufficiency. Abdominal techniques are contraindicated; the affected extremity (especially in leg lymphedema) should be treated only in sections to avoid too much venous blood and lymph fluid returning to the heart during the treatment. Lighter compression is necessary for the same reason.
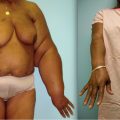
Lymphedema and obesity. Obesity contributes to the onset of lymphedema and often worsens the symptoms of existing lymphedema (see p. 309). In a 2008 study conducted by researchers at the University of Missouri-Columbia, it was suggested that the risk of developing upper extremity lymphedema following breast cancer surgery was 40%–60% higher in women with a body mass index (BMI) classified as overweight or obese, compared with women of normal weight. In their study, which included 193 breast cancer survivors, researchers also reported that the risk of lymphedema is especially high in over-weight or obese women who undergo cancer treatment involving the dominant side, or who experience postoperative swelling.
Excessive weight and obesity may also contribute to the onset of primary and secondary lymphedema involving the lower extremities. Excessive weight, especially morbid obesity, can have a negative impact on the return of lymphatic fluid from the legs; additional fluid volumes associated with obesity may overwhelm an already impaired lymphatic system. Direct pressure on lymphatic vessels by excess fatty tissue, impaired diaphragmatic breathing and decreased muscular function can also be factors contributing to the manifestation of lymphedema. Chronic venous insufficiency (CVI) is often associated with obesity. The increased burden on the lymphatic system in CVI can play a significant role in the manifestation of lower extremity lymphedema.
The progress of treatment of existing lymphedema may be seriously hampered in patients with a high BMI. With obese patients it is often difficult to apply bandages, especially in cases of lymphedema affecting the lower extremities. Furthermore, the compressive materials (bandages, garments) applied to the affected extremities have a tendency to slide in obese patients. Compression garments may have to be custom made, creating an additional financial burden on the patient.
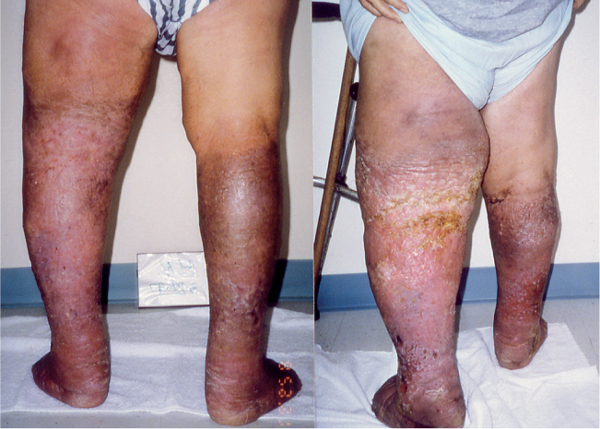
Fig. 3.22 Lymphedema in combination with venous insufficiency (stage 3), ulcerations, and lymphorrhea on both lower extremities before (right) and after (left) complete decongestive therapy.
Exercise—a very important aspect of the management of lymphedema—may be made difficult as well. Mobility problems associated with a high body mass index can affect the patient’s participation in treatment, and exercise protocols used in lymphedema therapy for the upper and lower extremities may have to be modified accordingly.
Weight management and proper nutrition are essential for successful long-term lymphedema management (see also Nutritional Aspects in Lymphedema later in this chapter).
Lymphedema and orthopedic problems. Symptoms associated with lymphedema and orthopedic pathologies will magnify each other and may even exacerbate the current presentation of either or both pathologies. Relevant combinations are “frozen” shoulder and upper extremity lymphedema or hip/knee problems associated with lymphedema of the leg. To interrupt the vicious cycle, it is necessary to address all involved pathologies. It is often advised to prioritize treatment according to the more limiting factors found during the assessment.
Lymphedema and venous insufficiency. Venous insufficiency contributes to the onset of lymphedema and worsens symptoms of existing lower extremity lymphedema (see Chronic Venous and Lympho-venous Insufficiency later in this chapter). The additional presence of venous ulcerations necessitates proper wound care (Fig. 3.22; see also Wounds and Skin Lesions later in this chapter). It is recommended that the lymphedema therapist incorporate the materials prescribed by the physician into the compression bandage.
Although venous insufficiency will benefit from CDT for lower extremity lymphedema, it is imperative to observe possible complications associated with venous insufficiency (thrombophlebitis). These complications may present a contraindication for the treatment of lymphedema.
Lymphedema and diabetes. Diabetes is often associated with dry skin, frequent infections, neuropathy, slowhealing wounds, and high blood pressure. To address these problems, the treatment protocol for lymphedema may be modified accordingly, with more emphasis on skin hygiene. If ulcerations are present, the incorporation of wound care into the protocol becomes necessary. As with venous ulcers, it is recommended that the lymphedema therapist use and incorporate the materials prescribed by the physician into the compression bandage.
Axillary Web Syndrome
Definition
Axillary web syndrome (AWS) is a condition that may develop after interruption to the axillary lymphatics such as axillary lymph node dissection, sentinel lymph node dissection, trauma, or an obstruction from cancer. It is a visible and palpable web of tissue that becomes taut with shoulder abduction. It is located in the axilla region and often extends distally along the anterior, medial upper arm toward the antecubital space and may extend as far as the base of the thumb. In patients with a thin body type, it may also extend proximally along the lateral chest wall (Fig. 3.23a). In a few cases, subcutaneous nodules have also been reported along the cord. It has the appearance of a tight cord of tissue being stretched underneath the skin (Fig. 3.23b) and is sometimes referred to as “cording.” Other terms used to describe AWS include Mondor’s disease, lymphatic cording, subcutaneous fibrous banding, fiddle-string phenomenon, lymph vessel fibrosis, lymphangiofibrosis thrombotica occlusiva, and lymph thrombosis. AWS is painful and can limit range of movement (ROM) of the shoulder, elbow, wrist and trunk. The cord tends to be more extensive in patients who have had axillary lymph node dissection compared with those who have had sentinel lymph node dissection. AWS should not be analogous with soft-tissue tightness, because many patients have soft-tissue tightness following axillary lymph node dissection but do not have AWS.
Fig. 3.23a, b
a Location of the axillary web syndrome.
b Axillary web syndrome of the left arm (multiple cords visible in the antecubital space).
AWS appears to develop more often in patients who have a thin body type. It is suggested the cord may be more difficult to detect in heavier-set patients because the thick layer of subcutaneous tissue may cover the cord. The subcutaneous fat tissue may also make it more difficult for the skin to adhere to the underlying tissue caused by the cord.
Physiology/Pathophysiology
It has been indicated that AWS is a variant of Mondor’s syndrome. Mondor’s syndrome is caused by a thrombosed superficial vein and presents as a cord on the chest wall, which is painful, tender, and causes skin retraction and pulling. AWS development is thought to be caused by an injury to the axillary lymphatics following axillary lymph node dissection, trauma, or an obstruction from cancer. An interruption of the lympho-venous channels causes a thrombosis and stagnation of the lympho-venous fluid, resulting in inflammation, fibrosis, and shortening of the tissue. Tissue biopsies of the cord taken from a small number of patients have indicated dilated lymphatics, a fibrin clot in the lymphatics, and venous thrombosis.
The reported incidence of AWS is variable ranging from 6%–72%. There are indications that AWS primarily occurs in the early postoperative period beginning ~1–5 weeks following surgery and resolves on its own within 2–3 months. Although its occurrence is most often seen in the acute stages following surgery, AWS has been reported to develop months or years after surgery. Lingering remnants of the AWS cord have also been observed beyond the 3-month timeframe following surgery.
Evaluation
Because there is postoperative delay in the development of AWS, the patient will typically have fairly normal ROM following surgery. Within a few weeks, the patient starts to experience tightening and pain in the arm which begins to limit ROM. The patient may come to the clinic with the affected arm in a protected position of shoulder protraction, internal rotation, elbow flexion, with wrist flexed and supinated because it is painful to let the arm rest by the side. Shoulder abduction is the most limited movement in most cases, but elbow extension is also often limited especially with arm abduction. The cord is taut and painful with palpation.
With a severe cord that extends to the elbow or base of the thumb, the cord will be easily detected by the attempt to position the arm at the side. The patient will not be able to extend the elbow or wrist fully and a cord can be visible and palpable in the forearm. If the cord is located in the axilla, the cord will become visible when the arm is abducted and a stretch is applied to the cord. In thin patients, a cord may be visible on the lateral chest wall at the end range of shoulder abduction and trunk sidebending. Some patients will report tension in the lateral chest wall similar to the cording symptoms felt in the arm and axilla even though a cord may not be observed. The reason the cord may not be visible in this area may be due to the presence of subcutaneous fatty tissue. A thorough assessment of all affected areas is indicated so that fragments of the cord are not missed.
Therapeutic Approach
From a therapist’s perspective, any indication of AWS should not be ignored. Treatment of AWS can rapidly reduce the pain caused by the tension of the cord and dramatically improve ROM and function. Without treatment, AWS could cause prolonged shoulder immobility which could lead to secondary problems such as altered movement patterns, poor posture, malalignment, muscle imbalance, impingement, frozen shoulder, soft-tissue tightness, and chronic pain.
An effective manual technique used to treat AWS is skin traction starting at the most distal portion of the cord and working proximally. It involves a gentle stretch on the superficial tissue over the cord. Using the palmar surface of the fingers, both hands are placed ~2–10 cm apart along the cord and a stretch is applied along the direction of the cord in the opposite direction. (Fig. 3.24). Once the patient no longer feels tension in the area, the arm is repositioned into further abduction to apply more tension on the cord. Feedback from the patient is imperative throughout treatment as the cord may become tight in another area as one area is relieved. Work the area of the cord where the patient indicates tightness. The elbow should stay extended throughout the progression into abduction. Wrist extension can also be applied to achieve tension on the cord.
Cord bending is another manual technique used to treat AWS. While the cord is tensed when the patient is positioned in a stretch, a perpendicular pressure is applied on the cord with the thumbs while bending the tissue in the opposite direction with the other fingers. Cord bending should be applied to the area of the cord where the patient indicates tightness. This technique can also be used in the pectoral region to stretch the tissue (Fig. 3.25).
The cords may break or release during treatment and a palpable and sometimes audible pop can be experienced. No adverse effects have been reported following the breaking of the cords though it is important to be cautious and avoid being overly aggressive in trying to break the cords until further research is obtained on the exact mechanics of the cord breaking. It is not known whether the noise is due to the actual cord or the supporting fibrous tissue around the cord breaking, or to another cause. If a cord does break, an immediate increase in ROM is experienced. To reduce anxiety, it is beneficial to explain to the patient that the cord may break and that he or she may hear and feel the cord release during treatment and to reassure him or her that this is a normal response. Gentle manual techniques are quite effective in helping resolve the cords without being overly aggressive.
If the cord extends into the arm, a gentle but effective approach to the rapid resolution of the cord is the short-term use of gradient compression bandaging. The application of low-stretch compression bandages achieving a gradient compression can be used for 1–3 days. Less compression is needed compared with regular lymphedema compression bandaging, therefore 2–3 compression bandages with appropriate padding are usually sufficient. If there is lymphedema involvement, the appropriate compression to treat the lymphedema is indicated. Like all bandaging, close monitoring and patient education is necessary to avoid complications. A compression garment is not as effective in resolving the cord.
Instruction in a home exercise program is dependent on the location and severity of the cord. Gentle ROM exercises should be initiated at the most distal portion of the cord first. A cord that extends to the base of the thumb can be quite painful; therefore thumb and wrist extension in addition to elbow extension may be all the patient can tolerate initially. Proximal ROM exercises (such as pendulum exercises and/or the finger walk on the wall) can be added as the cord resolves distally. The patient can be instructed in a self-skin stretch, similar to a nerve stretch, by placing the affected hand on the wall with the wrist extended, forearm supinated, elbow extended, arm abducted below 90 degrees and scapula depressed. The palmar surface of the unaffected hand can be used to apply a gentle proximal stretch on the cord (Fig. 3.26). This self skin-stretch of the cord can also be applied with more advanced stretches such as pectoralis and lattisimus dorsi stretches or other exercises indicated by therapist. A caregiver can also be instructed in manual techniques to stretch the cord to help increase the ROM.
It is important to focus on postural education throughout treatment. Myofascial release, craniosacral techniques, scar mobilization, joint mobilization, nerve glides, and strengthening can also be incorporated into the treatment as indicated by the therapist. Nonsteroid anti-inflammatory drugs (NSAIDS) and opioids may also be beneficial for pain management related to AWS.
Impact of Lymphedema on Quality of Life
Lymphedema affects psychological well-being and quality of life of people of all ages,16 cultures,17,18 and genders.19 Although the majority of research has been done in the area of breast cancer-related lymphedema3,20 and the highest proportion of patients living with lymphedema in the developed world may be survivors of breast cancer,21 studies show that patients suffering from either primary or secondary lymphedema experience a negative impact on their quality of life from this chronic disease.16,19,20,22–24
The association of quality of life and lymphedema has long been a concern, with research dating back over three decades.16,25,26 Primary lymphedema is reported to affect 1.15/100 000 people under 20 years of age.16 As far back as 1985, Smeltzer et al.16 reported findings from a longitudinal study at the Mayo Clinic of children and adolescents with primary lymphedema that led to recommendations that adolescents, in particular, be referred for psychological counseling.
A decade later, noting the absence of a validated tool for assessing quality of life among patients living with lymphedema, despite the general clinical awareness of such a problem, Augustin et al.19 developed and validated a tool for assessment of quality of life in lymphedema. They found that patients with primary and secondary lymphedema showed marked impairment in quality of life in all areas assessed (e.g., physical status, everyday life, social life, emotional well-being, treatment, satisfaction, and profession/household), compared with patients with early-stage venous insufficiency, and comparable reductions in quality of life compared with patients with venous leg ulcers.
In an area of greater investigation, researchers reporting quality of life outcomes among breast cancer survivors with lymphedema detail impairments in both physical and psychological aspects of life.27 For example, McWayne and Heiney28 and Collins et al.29 reported that psychological problems (e.g., frustration, distress, depression, and anxiety) are associated with the presence of lymphedema among breast cancer survivors. In addition, lymphedema may compromise the normal activities of daily living (e.g., sleeping, driving, carrying items, household chores, occupational responsibilities, dressing, or gardening and other leisure activities),30–33 domestic role,35 and family responsibilities.32 Survivors with lymphedema experience more symptoms (increase in arm and shoulder size; tighter fitting clothing, sleeve cuff, and jewelry; limited elbow function; arm/hand weakness; loss of sleep secondary to arm discomfort; tenderness; swelling; pitting; blistering; firmness/tightness; heaviness; stiffness; aching; breast swelling) than those survivors without lymphedema.22–24,35 While breast cancer survivors report that they are most fearful of a cancer recurrence, it is noted that their second greatest fear is that they will develop lymphedema.36,37
Konecne and Perdomo38 reported that lymphedema could lead to functional limitations for elderly individuals (e.g., decreased ability to reach, lift, push, pull, twist, carry, shove, and grasp), which in turn could affect their quality of life. As people living with primary and secondary lymphedema grow older, the impact on quality of life may burgeon.39
This chapter focuses on the personal impact on quality of life when living with lymphedema based on qualitative studies of individuals and families and the objective impact on quality of life in areas such as function and finances. Personal quotations (Armer, unpublished data) and studies that are selected for review22,40 reveal the universal and widespread impact of this chronic condition.
Personal Views of Lymphedema across the Globe
It’s like giving birth. Everybody can tell you it feels like this and this, but until you experience it yourself, you don’t know…
If you’ve got children and you’ve got a husband, you’ve got to be up. There’s no time to be sick… It does influence relationships, especially with your husband… My husband likes a good looking woman with breasts and a nice body and everything. So it has been hard work for me to, to survive this whole thing…
I think at first… you try to have a lot of hope that this thing… is a temporary sort of situation. And I think that, um, the disappointment, with time and as you realize it’s not going to go away, something that you have to cope with for the rest of your life, so it is a disappointment.”–V, breast cancer survivor and professional business woman, living with lymphedema, South Africa Since going through breast cancer 5 years ago and developing a swollen arm my doctor says [it] will only get bigger… caring for my family has been very difficult…
I fight cancer, through my weakness and through my struggle… I struggle…
Sacrifice… I am a humble person. I am grateful for everything, everyday. I am grateful…
There are other times when I think about my thick arm because of the weight of the arm. But then I have to put it out of my head… there are other things to worry about, a decent place to live, running water…” – E, breast cancer survivor and hourly domestic worker, living with lymphedema, South Africa
“Going through treatment for breast cancer (41 years ago) was nothing compared to all these (39) years with lymphedema.” – R, breast cancer survivor and retired secretary, living with lymphedema, Midwestern United States
These quotations reveal the personal dimensions of living with lymphedema following breast cancer treatment in two very disparate parts of the world, with comments resonating with the impact on quality of life among individuals of vastly different ages and cultures. It is important that health professionals hear the voices of the individuals they treat who are living with the challenges of lymphedema.
Impact of Minimal Limb Volume Change on Quality of Life among Survivors with Lymphedema
A recently published prospective cohort study40 assessed symptoms, quality of life, and health status among women undergoing surgery for breast cancer. Serial upper extremity circumference, perometry (Juzo, Cuyahoga Falls, OH) volume, and symptom reports were assessed every 3 months for 1 year, and every 6 months for the next 18 months. A model adapted from Wilson and Cleary42 and Ware43 guided the analysis suggesting that health-related quality of life such as specific health measures (symptoms and limb volume change) and generic health measures (function and quality of life) are among the best predictors of future health outcomes (such as expenditures, response to treatment, work productivity and disability, and mortality). Analysis revealed that although functional status tended to decrease slightly but nonsignificantly with increase in volume change from 5% to ≥15%, symptoms increased significantly with volume increase and quality of life decreased significantly even with mild limb volume increase (Fig. 3.27).
These findings underscore the importance of assessing symptoms, limb volume, function, and quality of life among individuals living with lymphedema. They reveal the association of even mild increase in limb volume with decreased quality of life. These findings support the imperative for early, effective, evidence-based interventions for those meeting the diagnostic criterion for lymphedema.43
Fig. 3.27 Graph of symptom score, function (36-Item Short Form Health Survey [SF-36]), and quality of life (Functional Living Index-Cancer [FLIC]) by categories of limb volume change. (From: Cormier JN, Xing Y, Zaniletti I, Askew R, Stewart BR, Armer JM (2009). Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42 (4):161–175. Reproduced with permission of Lymphology).2
Economic Impact of Lymphedema for Survivors
A team headed by a health-economist researcher conducted a study to estimate costs associated with lymphedema by comparing the total cost of insurance claims for survivors with breast cancer lymphedema with that of a matched cohort of breast cancer patients without lymphedema.40 Claims data from a national employment-based database of 1877 individuals with an average age of 48.8 years and a 9.6% lymphedema occurrence rate were used. There was a 3:1 match of breast cancer controls to breast cancer-lymphedema patients, matched by breast cancer treatment characteristics (mastectomy, lumpectomy, node dissection; chemotherapy; radiation therapy). A regression model was performed to compare the risk of cellulitis or other infections in survivors with and without lymphedema and to estimate costs associated with lymphedema.
The research findings of Shih et al.40 revealed that those with lymphedema had a statistically greater risk of submitting health claims because of cellulitis or lymphangitis and other complications.40 Furthermore, 2-year estimated health claims costs were more than $22 000 greater for those survivors with breast cancer-related lymphedema than for those without lymphedema, with approximately $13 500 of these excess claims not being related to cancer treatment.40 As related specifically to quality of life, breast cancer survivors with lymphedema utilized more psychological counseling services than breast cancer survivors without lymphedema.
It is important to note that these data are drawn from a database of employed adult women with health insurance, and so these data represent the more well-represented, higher insured, less vulnerable population with and at risk for lymphedema; other uninsured or underinsured groups may have greater complications and health costs, and subsequently greater impact on their quality of life.
Impact of Lymphedema on Family Life
Findings from a series of ethnographic studies by Radina and Armer32,33,44 reveal information on coping with chronic illness and the impact of lymphedema on women’s family roles; resiliency among women with post-breast cancer lymphedema and their families; and the survivor’s transition from caregiver to care-receiver.
Coping with Chronic Illness: Impact of Lymphedema on Women’s Family Roles
The first study investigated the effect of lymphedema on women and their families regarding task completion and family functioning using the Family Adjustment and Adaptation Response Model.32 For example, following the development of lymphedema, women may no longer be able to move furniture, carry in groceries, and care for children or aging parents as they used to. Families who were more flexible in modifying daily tasks and who had pre-existing resources for coping with stressors were found to have more positive outcomes than did those families who were rigid and coped poorly with stressors.
Resiliency among Women with Lymphedema and their Families
The second study was guided by the Resiliency Model of Family Stress, Adjustment, and Adaptation.44 This investigation into how lymphedema onset and its related stressors impact women and their families found three particular stressors: 1) required modification of daily tasks; 2) lymphedema as a reminder of breast cancer; and 3) frustration with medical professionals.
Transition from Caregiver to Care-Receiver: Functionally Autonomous Survivors with Lymphedema
The third study explored women’s experiences of off-time life course transitions from being the caregiver to becoming a care-recipient as a result of post-breast cancer lymphedema.44 Women in the prime productive years of work and family unexpectedly making the transition to the role of receiver of care, an often abrupt change which was out of step with age and developmental peers. Two dominant themes were revealed: 1) not wanting to be a burden; and (2) desiring to live an independent lifestyle. Even in the presence of family and friends willing to help, women wanted to continue to live autonomously as they had done before.
These qualitative studies provide insight into the impact of lymphedema for survivors and their families. Together, they provide evidence which corroborates and further illuminates the findings of Shigaki et al. (in review)34 and others30,31,33 about the impact of lymphedema on both the individual and the family in compromising the domestic role and activities of daily living. Radina and Armer44 found that families were in large part responsible for the individual’s adaptation and adjustment to living with lymphedema. These studies suggest that interventions within the family could increase the resiliency of patients with chronic illnesses. Therefore, health professionals should be concerned with the entire family, or even community, rather than just the individual.
Coping with Lymphedema
A qualitative study by Heppner et al.45 examined the stressors associated with lymphedema following breast cancer and the roles of coping and social support in response to the stress. This study used intensive semistructured interviews and consensual qualitative research methods. Survivors with lymphedema experienced a broader array of stressors than previously reported in the literature. These findings included the following themes: (1) lymphedema impact is pervasive; and (2) approach coping rather than avoidant coping is reported to be beneficial.
Stressors associated with lymphedema included (with scale of frequency of reports; number representing cases supporting the theme; and the most frequently reported are in bold):
• Concerns about (and excessive time demands related to) maintenance of daily activities (10)*
• Physical symptoms and pain associated with lymphedema (10)*
• Negative emotional and cognitive reactions (9)
• Lack of concerns and caring of health care providers (9)
• Concerns regarding their prognosis (7)
• Attractiveness and sexual issues (6)
• Negative social support (6)
• Lack of adequate health insurance coverage (6)*
• Stress and anxiety in partners/children (5)
• Absence of social support (2)
• Cultural-related stressors (1)*
Coping strategies to manage lymphedema-related stressors were reported as:
• Actively sought information or treatment options (10)
• Learned physical strategies to manage lymphedema symptoms (10)
• Accepted the limitations associated with lymphedema symptoms (10)
• Focused on the positive aspects of life (10)*
• Used spiritual/religious methods (9)
• Openly talked and educated about lymphedema (9)
• Maintained leisure and recreational activities (6)*
• Used ineffective coping methods (5)
• Impact of their racial and socioeconomic backgrounds on coping (1) *
Among social support and social resources for coping with lymphedema-related stressors were:
• Opportunity to nurture others (10)*
• Reliable alliance of others besides partners (9)
• Reliable alliance of partners (8)
• Concern and support from health care providers (7)*
• Spiritual support from others (5)
• Maintaining the status quo (3)*
This qualitative study examining coping strategies and stressors reported by breast cancer survivors provides guidance for health professionals in assessing coping and stresses experienced by those living with lymphedema. Together, these qualitative studies lay the foundation for research designed to develop and evaluate interventions to strengthen family function, enhance coping, and lessen the stresses associated with this chronic condition.
Reframing the Situation
The literature furthers understanding of the impact of lymphedema on quality of life among those living with this chronic condition.22,32,40,44 A final quote captures the essence of the possibility of reframing what may have proved to have been both an unexpected and devastating life event, whether the emergence of primary or secondary lymphedema.
“It [lymphedema] isn’t minor, but the gratitude is that I have learned so much about my body. Because out of negative experiences can come positive, and I suppose that’s one of the ways I cope. I try to look for what positive can come from this. And if you can see positives, you can cope a little better. It doesn’t become so dramatic.” – U, participant in urban setting in Midwestern US, data from Heppner et al.45
* General 10 or more cases; typical 6–9 cases; variant < 6 cases
Research studies such as those reviewed here provide insight into the personal nature and challenges of living with lymphedema and the foundation for evidence-based interventions to enhance quality of life for individuals and families living with lymphedema.
Diagnosis of Lymphedema
Lymphedema is a disease without a “gold standard” or single unifying diagnostic criterion that is reproducible, universally accepted, and generalizable. Despite the fact that the disease is dynamic, the current diagnostic criteria are based almost solely on physical examination findings. Due to the lack of a universally accepted diagnostic standard, the incidence of lymphedema will inevitably vary with the method used to define its presence.
The Agency for Healthcare Research and Quality (AHRQ) Technology Review of Lymphedema stated: “Is there any ‘gold standard’ method to formally grade or measure the severity of lymphedema? Based on the evidence in the extracted studies, there does not appear to be a gold standard to formally grade or measure the severity of lymphedema.”46
Most studies have focused on secondary lymphedema, primarily breast cancer-related lymphedema (BCRL), and there is relatively little literature on lower extremity, truncal/breast, genital, abdominal, head and neck, and primary lymphedema.
Various Approaches to the Diagnosis of Lymphedema
1. “The 2-cm rule”: this applies to a circumferential measurement discrepancy between two limbs, and its sensitivity and specificity are directly related to the number of measurements obtained and whether they are used to create a limb volume. Armer et al. found that using a single point discrepancy, on women followed for 5 years, resulted in a 91% incidence of lymphedema. The National Lymphedema Network (NLN) position paper on Early Detection of Breast Cancer-Related Lymphedema has stated that a single 1 cm increase from baseline should result in a 1-month follow-up and a 2 cm increase should result in treatment referral. Clearly, this interlimb discrepancy diagnosis cannot be applied to nonlimb lymphedema.47
2. Limb volume measurement: This can be obtained from circumferential measurements, perometry or volume displacement measurement. Circumferential measurements can be used to calculate arm volumes with either a 2-point system: frustum sign method (volume of a truncated cone) or disk model method (summed truncated cones). The significant limb volume discrepancy for diagnosing lymphedema has not been clarified: 5% volume increase has been found to have a 91% sensitivity rate, versus a 10% increase, which corresponded to a 49% sensitivity.48,49 Currently, there is a website that supplies an arm volume calculator: http://www.armvolume.com/(accessed June 7, 2012); it utilizes six measurements at 7-cm increments. One study measured accuracy of limb volume calculated by incremental measurements, and concluded that while 4 cm measurements were accurate for arm volumes, leg volumes could be obtained with either 4-, 8- or 12-cm incremental measurements.50
3. Physical examination: Stanton et al.51 used perometry and physical examination and found, for breast cancer patients whose volume measurements were within normal limits, that examination discovered early signs of lymphedema: decreased visibility of veins, smoothing of the contours of the medial elbow region, increased skin and subcutis thickness on palpation, and pitting edema. Limitation of edema to focal regions was noted, for example, hand and wrist only or upper arm. Stemmer’s sign, fixation of digital skin, is a timehonored sign of lymphedema.
4. Symptoms: patients will report symptoms of swelling, warmth, heaviness, tingling, tightness, and/or skin changes. Armer et al. have developed the University of Missouri Lymphedema and Breast Cancer Questionnaire (LBCQ) survey to assess BCRL. Symptoms can precede overt swelling and latency stage lymphedema may only present with symptoms or subtle physical findings as mentioned above.
5. Newer technologies: bioimpedance spectroscopy has been marketed specifically to diagnose latent lymphedema, stage 0—prior to physical changes. The device measures extracellular fluid, but requires accurate use and sequential measurements and is not useful in patients with either swelling or fibrosis. As early diagnosis and prospective monitoring of lymphedema have been proved to minimize BCRL, bioimpedance is a measurement modality, which combined with a full assessment of the patient, can aid in detecting early lymphedema. The device’s literature states that it is not intended to diagnose or predict lymphedema, yet it has been proposed that it be utilized in this manner.52
6. Imagining: Per the AHRQ review: “The validity of ultrasound, lymphoscintigraphy, CT scan, or MRI was evaluated in four studies. There is little evidence for the validity of these tests owing to the limited number of studies, small sample sizes, a questionable reference standard in one study, and questionable means of scoring lymphoscintigraphy in two studies.”46
7. Nonextremity lymphedema: the NLN position paper on BCRL suggests that for truncal and breast lymphedema, patients be examined for “objective” evidence/visualization of swelling in the chest or trunk, and symptoms referable to the area. Williams states, “there is no fully validated method to assess these areas,” despite the possible incidence of truncal/breast lymphedema in up to 70% of treated patients.53
8. Head and neck lymphedema: Patients treated for head and neck cancer have been assessed externally using the Földi Scale, and internally with endoscopy and found to have a high incidence of lymphedema—up to 50%.54
9. Lymphedema rating scales: The Földi Scale; the Common Toxicity Criteria v3 lymphedema; the LVF Location, Volume, Fibrosis Scale by RG Kasseroller; International Society of Lymphology Staging (below). Most scales rely exclusively on volume, which is of little value in bilateral or nonlimb lymphedema.
International Society of Lymphology (ISL) Lymphedema Staging
ISL Stage 0
A subclinical state where swelling is not evident despite impaired lymph transport.
This stage may exist for months or years before edema becomes evident.
ISL Stage I
This represents early onset of the condition where there is accumulation of tissue fluid that subsides with limb elevation. The edema may be pitting at this stage.
ISL Stage II
Limb elevation alone rarely reduces swelling and pitting is manifest.
ISL Late Stage II
There may or may not be pitting as tissue fibrosis is more evident.
ISL Stage III
The tissue is hard (fibrotic) and pitting is absent. Skin changes such as thickening, hyperpigmentation, increased skinfolds, fat deposits, and warty overgrowths.
Yet, with any evaluation, a thorough patient history is vital for making the diagnosis, and often before lymphedema manifests with physical symptoms, there are subtle symptoms and history that help in making the diagnosis.
History
The history should be taken to assess both primary and secondary lymphedema and other medical conditions that cause swelling.
There are the classic cardinal signs for history of present illness: location, quality, radiation, intensity/severity, onset, aggravating factors, alleviating factors, and associated symptoms.
The pattern of illness/symptoms should be obtained. Lymphedema is a dynamic process. The nature of the onset should be documented: sudden, insidious, or provoked by an incident such as trauma or infection. The nature of the symptoms should also be documented: wax and wane, progressive, or provoked by behaviors such as exercise, heat, or prolonged standing.
The pattern of swelling gives important clues as to the diagnosis. In primary lymphedema, the swelling typically begins distally—usually in the toes, although rarely hands may be involved—and progresses proximally. Lipedema is a disorder of subcutaneous fatty tissue, tends to affect women and disproportionately involves the lower half of the body while sparing the area below the ankles. In lipedema the affected areas are typically hypersensitive to touch and pressure. Secondary lymphedema occurs in the quadrant at risk after disruption of the regional lymphatics due to surgery, radiation, injury, and/or infection. The swelling of secondary lymphedema can start in any portion of the quadrant. In lymphedema secondary to venous insufficiency, there will be a history of varicose veins, venous stasis changes, and/or deep vein thrombosis, and the swelling usually begins distally and initially resolves with elevation.
Patients should be asked if there is a family history of swelling and limbs that do not reduce despite weight loss. Body habitus and familial body habitus give important clues as to lipodema and primary lymphedema.
Foreign travel history is important to assess filariasis.55
Disruption to the lymphatic system should be assessed: history of trauma, surgery, radiation, chemotherapy, venous thrombosis, immobility, and history of skin infections.
Secondary causes of swelling should be assessed, such as congestive heart failure, renal failure, venous insufficiency, varicosities, hepatic insufficiency, hypothyroidism, neuropathies, reflex sympathetic dystrophy, and any paralysis.
Additionally medications can cause peripheral edema, so a thorough history of both prescribed and over-the-counter medications should be obtained.
A history of symptoms that are associated with swelling should be obtained: heaviness, achiness, tight clothing or jewelry, noticeable swelling, changes in the contours of the body, obscuring of bony or tendon landmarks, marks left by clothing or other pressure, warmth, color changes of the skin, numbness, paresthesias, and the temporal pattern of these symptoms. A validated questionnaire for breast cancer patients has been developed by Jane Armer PhD, the LBCQ questionnaire.49
As with any patient, a full history of present illness, past medical history, and social history—with attention to occupation, functionality, exercise, family history, and review of systems—should be obtained. Screening for mood disorders should also be carried out. Additional special questionnaires for assessment of function, pain, and symptoms should be utilized.
A thorough review of systems should be obtained bearing in mind the area where lymphedema is suspected.
As always, symptoms of pain should be documented and scored.
Physical Examination
General
Vital signs, including body mass index, should be obtained; weight should be noted at every visit. General observation of gait, balance, use of limbs should be noted.
A general physical examination should be performed, within the health care provider’s area of expertise—clearly this will vary by training, but ideally the most complete physical examination that the practitioner is qualified to perform should be obtained. For example: for head and neck lymphedema, endoscopic evaluation is preferred, as well as a functional swallowing evaluation.
Assessment of cardiac, pulmonary, hepatic, dermatological, neurological, and musculoskeletal systems should be performed.
Specific for Lymphedema
The patient should be examined in all four quadrants of the body, with attention to noticeable swelling, obscuring of tendon and bony landmarks, skin examination, surgical scars, musculoskeletal function, and neurological examination. The skin examination should focus on changes in the cutis, thickening, fibrosis, color changes, skin lesions, a full nail examination, varicosities, venous stasis changes, and evaluation of all wounds. Presence of Stemmer’s sign should be documented.
The skin changes associated with progressive lymphedema should be assessed: thickening, papillomatosis, hyperkeratosis, lymph vesicles, and/or fistulas.
In the breast, skin thickening can present as a “peau d’orange” pattern.
As described above, a quantitative measurement of the patient should be obtained, if possible: interlimb volume for limb edema obtained as calculated volumes, perometry measurements or water displacement measurements.
Additionally, to assess general extracellular fluid, serial bioimpedance spectroscopy measurements can be obtained—bearing in mind that the L-Dex is not intended to diagnose or predict lymphedema (see manufacturer’s information 10)—following consistent guidelines that involve an empty bladder, avoidance of caffeine and alcohol, and lying prone for an adequate period. Bioimpedance spectroscopy measures extracellular fluid and can assist in the diagnosis of lymphedema—primarily at the latent stage. The device is not reliable once there is skin fibrosis or swelling (ImpediMed correspondence.)
For nonlimb suspected lymphedema, the patient should be examined for overt swelling—pitting or nonpitting, changes of the skin and subcutis, and use of photography may be helpful to document external physical examination abnormalities, as in breast/truncal, genital, abdominal, and head and neck edema.
Lymphedema is edema, just composed of lymphatic fluid. Patients will present with edema that is due to congestive heart failure, nephritic syndrome, hepatic insufficiency, and vascular insufficiency. They can have coexisting lymphedema as well if the lymphatic system is damaged. Additionally, morbid edema can cause lymphedema due to obstruction.
Reassessment
As lymphedema is a chronic condition, reassessment is crucial.
A focused history of symptoms and modalities used to treat the condition should be obtained.
Weight and height, and BMI should be obtained, as well as current medications and interval medical conditions should be documented.
Symptoms and pain should be assessed, as well as body function.
Quantitative measurements should be documented, if applicable. For limb volumes, a 3% increase from a “normal” baseline is considered indicative of subclinical lymphedema and a 5% increase is considered both sensitive and specific for lymphedema. As Stanton has documented, observation of subtle changes may indicate clinically significant disease, even in the absence of volume increases. If bioimpedance is used as an adjunctive measurement, serial measurements with close attention to accuracy should be obtained: the manufacturer states that “L-Dex values of above +10 or increase of 10 may indicate lymphedema.” (http://international.l-dex.com/what-is-l-dex/, accessed June 7, 2012)
The concept of a treated chronic disease should be employed: a patient who has responded to CDT and compression may subsequently present with reduced limb or body volume and reduced symptoms, but as lymphedema is chronic, this documents a reduction in the severity of his or her disease and possibly a regression of his or her ISL stage, and is not an end point, but merely an excellent response to treatment.
Medical Education about Lymphedema
Unfortunately, the extent to which the lymphatic system is studied in medical school is typically observation during gross anatomy, and discussion of enlarged lymph nodes in pathophysiology. The topic of lymphedema is rarely, if ever, discussed. The majority of medical school graduates will not have sufficient medical education to possibly even be aware of the disease, and they will have insufficient education to diagnose it.
Postgraduate medical education tends to ignore lymphedema as well; there are virtually no questions on it in board examinations, nor is it featured in continuing medical education.
Even in specialties relating to areas where secondary lymphedema is a side effect of treatment, medical oncologists, surgeons, and radiation oncologists do not routinely receive education on the disease, diagnosis or treatment of lymphedema.
A recent study by Kaiser Permanente, of practicing physicians in a staff model health maintenance organization (HMO) setting, documented that medical oncologists and surgeons, and female physicians in general were better informed about BCRL, yet only 44% of physicians who treated women with breast cancer had ever made a lymphedema treatment referral.56
A personal observation: attending a survivorship conference sponsored by Harvard Medical School at a major cancer center, I spoke to the medical director of the Livestrong Clinic, whose mission statement included treatment of lymphedema, and this director was unaware of how to treat lymphedema. When contacted, their physical therapy department had hired lymphedema therapists, but did not know their level of training, and proposed making lymphedema a “sentinel event” which is synonymous with significant medical error. Additionally, the published information on this illustrious institution’s website on lymphedema contained many factual errors. Despite contacting the medical director of the institution, the website has not been updated, and subsequent survivorship conferences by this institution have not addressed lymphedema.
Case Study
A 58-year-old woman was treated for breast cancer with breast conservation and radiation. It is now 5 years since her cancer diagnosis and she has completed 5 years of tamoxifen therapy. Approximately 3 years ago, she underwent a course of complete decongestive therapy for lymphedema of the left, treated, arm and wears compression garments. She works in a clerical position and notes that she has tingling and aching in the arm, which is worse at night and often wakes her from sleep.
On examination, her arm has focal swelling at the proximal portion with thickening of the skin, obscuring of veins, and the skin is lighter in appearance. This area of thickening measures 2–3 cm wider than her untreated arm, but the other parts of her arms are equal in size, and there is no visible swelling in the dorsum of her hand, nor obscuring of tendons.
On examination of her treated breast, the skin is thickened, with prominent pores and still has residual radiation tanning.
The “red flags” in this case are the history of lymphedema, as the greatest predictor of progressive lymphedema is a history of mild lymphedema,57 the history of axillary surgery and radiation, and the symptoms of aching and tingling.
This patient most likely does not have a 10% volume increase in the affected arm, but clinically she has breast and truncal lymphedema and limb lymphedema with fibrosis and would benefit from treatment of her breast and trunk with manual lymph drainage (MLD) and compression garments as well as treatment of her focal area of fibrosis; and as her symptoms are worse at night, showing her how to apply bandages and advising her to wear different night garments might help towards reducing the swelling, fibrosis, and symptoms in her arm. She was discharged from lymphedema therapy without follow-up and at over 5 years out from breast cancer treatment, has most likely been discharged from many of her physicians. Yet her risk of lymphedema and progression of lymphedema is lifelong.
Summary
There is no single, unifying diagnostic criterion for lymphedema. Current diagnostic criteria tend to focus on limb lymphedema.
It should be realized that without unifying diagnostic criteria for lymphedema, there will be inherent variations in diagnosis, reported incidence, and prevalence of the disease.
Lymphedema is a dynamic process and reliance solely on a physical examination for diagnosis could result in disease being missed.
A thorough history is crucial, as is obtaining some form of objective measurement that is consistent to your practice. Lymphedema can be present even without a limb circumference discrepancy of 2 cm. Symptoms can precede and coexist with clinically apparent lymphedema. Physical examination results can fluctuate with treatment, provoking conditions and comorbidities.
Lymphedema can coexist with lipedema, venous insufficiency, congestive heart failure and other medical comorbidities.
Reliance on a single modality to diagnose lymphedema could result in disease being missed: patients should be assessed by symptoms, history, a physical examination and objective measurements.
Medical education is often lacking in lymphedema, at all levels of medical providers, and at all stages of their clinical careers.
Evaluation of Lymphedema
Accurate and timely diagnosis of lymphedema is essential so the appropriate amount of treatment can be applied in a prompt, cost-effective, and minimally intensive fashion. Treatment requires adequate management as soon as the condition is diagnosed. Lymphedema is a dynamic condition regardless of the stage. Managing a latency stage is vastly different from managing a severe stage III lymphedema, yet both are chronic and require lifelong care. Different levels of intervention are required according to the stage, severity, and complexity of lymphedema.58 Certified lymphedema therapists must embrace this more evolved approach toward managing lymphedema in all of its manifestations and acknowledge the variety of presentations encountered at the initial evaluation. These presentations can be divided into three distinct categories: (1) patients presenting once objective signs of lymphedema are present, (2) patients without objective signs who complain of subtle sensory changes in the affected area, and (3) patients who present pre-operatively to establish a baseline measurement prior to any surgery that will remove lymph nodes or who have a known risk of developing lymphedema.
A comprehensive evaluation process requires adequate time to perform a complete systems review, yet should be focused toward the presenting symptoms and/or diagnosis. The evaluation will provide the necessary data for differential diagnosis, to quantify and/or stage the condition, and to determine if complete decongestive therapy (CDT) or parts of CDT are indicated for treatment. It is usually the first time a patient has met a health care provider who is a specialist in the field of lymphedema, and the patient often lacks basic information about the condition. Patients may not realize the relevance of their entire medical and/or surgical history, which, along with their personal history, often reveals the subtle and progressive onset of their lymphedema or the potential to acquire lymphedema. In all cases, a thorough evaluation performed by the therapist can clarify the medical diagnosis and establish the rehabilitation diagnosis as a basis for skilled therapy services. Patients may be cleared for therapy, but many referring physicians may not realize the systemic effects that complete decongestive therapy (CDT) and its component parts has on the human body. The evaluation can also prevent unnecessary or unsafe treatment, by recognizing contraindications and/or precautions for treatment. Patients will occasionally present to the lymphedema clinic with an inaccurate diagnosis on the referral, as any swelling or edema that looks severe is dubiously termed lymphedema. To be accurately diagnosed as lymphedema, there must be an abnormality of the lymphatic system, whether acquired or developmental, that ultimately results in a mechanical insufficiency. When no clear cause for edema can be established after a thorough evaluation process, it is incumbent on the therapist to contact the referring physician for further medical work-up and consultation.
A thorough evaluation allows the therapist to identify impairments in function, determine the rehabilitation diagnosis, determine the prognosis for treatment, and develop a comprehensive plan of care. It will also assess education style, barriers to care, and the need for other medical treatments for concomitant conditions. The plan of care will include setting short-term and long-term goals, selecting all appropriate interventions (such as bandaging, MLD, compression garments, remedial exercise, and self-care), and developing a comprehensive discharge plan. It is imperative during the initial evaluation that the therapist determines the need for skilled care and confirms that the patient will have the ability to be independent with the help of his or her discharge plan. Otherwise, treatment may not proceed until the necessary caregiver(s) or assistance are in place. Similar attention must be given to the patient’s ability to obtain the necessary supplies and garments. If no clear source of insurance or self-pay option exists at the time of evaluation, then this needs to be arranged prior to initiating treatment. A therapist should not get midway through the intensive phase of decongestion, only to discover that the patient is not able to obtain the necessary garments as this would render the treatment useless. Many patients have significant financial restrictions that limit their ability to get appropriate compression garments. A plan must be in place prior to initiating treatment.
Prior to the actual evaluation, it is important for the therapist to get as much information as possible about the patient’s condition. This will help focus the evaluation as the therapist will have a better understanding of the extent of the lymphedema and the impact on the patient’s life. This can be accomplished by having all new patients fill out a pre-treatment lymphedema questionnaire similar to one used by the National Lymphedema Network.59 At the least, a simple form assessing functional status, work status, caregiver status, available social support, living situation, and transportation status will offer the therapist significant insight into the patient’s abilities and resources. Other options include using validated quality of life measures such as the LYMQOL,60 the Lymph-ICF,61 the Lymphedema and Breast Cancer Questionnaire (LBCQ),62 the ULL-2763 for upper limb lymphedema or the FLQA-I19 for arm or leg lymphedema. The use of such disease-specific tools may benefit the clinician by providing outcome measures to assess the effectiveness of treatment and will aid in the clinical assessment.60 These tools are often available directly from the researchers, but may have conditions placed on their use.
Clinics may want to develop a simple pre-evaluation questionnaire to assist their staff with triaging and setting up lymphedema evaluations based on the probable stage, severity, and complexity of lymphedema. It is important to note the time since onset, current symptoms, and known risk factors. Many clinics have long waiting lists to see patients with advanced stage chronic lymphedema for full intensive phase CDT treatment. A patient with stage 0/I lymphedema who is having sensory changes or preclinical symptoms of lymphedema cannot wait 1–2 months to be evaluated. There must be a mechanism in place to trigger the early intervention evaluation in a time-sensitive fashion, so prompt treatment of early-stage 0/I lymphedema can be initiated. Recent studies have demonstrated that upwards of 80% of lymphedema is not diagnosed until stage II.64 Studies have also shown that mild breast cancer-related lymphedema is a predictor of more severe lymphedema, and if left untreated ~50% of cases will progress to more severe lymphedema in 5 years.58 Similarly, a study found almost 80% of women mentioned symptoms of lymphedema to their health care provider, yet only 47% received treatment. The majority of patients receiving treatment were already latestage, having moderate to severe symptoms.64
The patient evaluation includes both subjective and objective components that will be combined to form a comprehensive assessment of the patient’s condition. Földi stated, “Medical histories, inspection, palpation, and percussion are increasingly neglected, and diagnoses are based on laboratory data. What is lost is an understanding of the interconnections.”65 The advantage of having a thorough lymphedema evaluation by a specially trained clinician is that he or she has the capacity to make the interconnections and ensure that proper treatment is provided based on a full understanding of the patient’s condition. The first few minutes of the evaluation yields critical information about the patient’s situation. How did the patient ambulate into the room? Is the patient demonstrating any signs of physical exertion? Is he or she sitting on the chair or on the treatment table with appropriate posture? Is someone with the patient for support? Observation starts the moment one sees the patient for the first time.
It is often helpful to begin the evaluation when the patient is still dressed by asking him or her about the history of the onset and development of the edema. The patient should be thoroughly questioned about the course of the swelling, going as far back into his or her history as required. Did the swelling develop slowly or rapidly? Was the presentation distal or proximal? Is there pain or discomfort? Does the patient have a known cause for the edema and how old was he or she when it was discovered? Is there a family history of swelling? Did the patient ever have an infection related to the swelling? If so, how many infections has he or she been treated for? Where was the infection located? What treatment(s) did the patient receive? Was he or she hospitalized for the infection? Is the patient currently under any prophylactic care for infections?
Allowing some time to establish a clinical relationship is important, as it allows the therapist time to educate the patient on how the lymphatic system works as they progress through the evaluation process. This will help the patient understand why the therapist must evaluate the entire body and why the patient needs to put on a gown for both evaluation and treatment. A thorough review of past medical and surgical history is required at this time. It is a good time to rectify the patient’s medication list, as it should match the reported health history and be assessed for common medications that cause edema. Patients often forget about very important medical conditions and/or surgical history. Recent diagnostic tests, laboratory work, and medical treatments should be noted. Any prior treatment, including self-care for their edema should also be noted.
Pain must be assessed and documented along with all other important baseline vital signs. The onset, location, duration, and description of pain should be noted along with factors that change the level of pain. Many patients will try to differentiate between pain and discomfort. The therapist should not judge the description or challenge the patient on whether lymphedema is or is not painful, but simply use a validated measure to document it. When there is no clear reason for the pain, further medical evaluation may be needed, particularly in the patient with a history of cancer treatment.
The initial evaluation also serves to educate patients on general treatment protocols within the lymphedema clinic. Patients should be shown how to put on the gown and drape their body with a sheet if required. Patients need to be educated on why they should keep their shoes off the treatment table and their bare feet off the floor. The therapist may also assess functional activities such as the ability to put on or take off shoes, general mobility, and self-care. Timing how long it takes patients to change into a gown can give a general indication as to their self-care status, as can their ability to accomplish this alone or with assistance. At this time, patients should be offered a chaperone, if they would like one or if institutional policy requires that one be present. This is particularly important in the case of genital lymphedema assessment. If the patient is a minor, then the parent or legal guardian must be in the room for the entire evaluation regardless of diagnosis.
The upper extremity patient should be in a patient gown that leaves the entire thorax above the waist/transverse watershed visible. Having the gown open toward the posterior thorax allows for more discrete examination to begin on the thorax. Similarly, the lower extremity patient must be in a gown that leaves the entire region below the transverse watershed visible. Patient education occurs throughout the evaluation as the therapist explains many concepts to the patient, such as why the contralateral limb must be assessed, and why unaffected tissue needs to be inspected. Education with anatomical depictions of the different territories, watersheds and anastomoses is very important so that the patient has adequate understanding of how treating unaffected tissue influences affected tissue.
The appearance of the involved limb(s), body part, and/or quadrants along with observations on the unaffected areas should be noted first, gaining an appreciation for the symmetry or asymmetry of the body region involved and the general condition of the skin. Are any of the areas of lymph drainage affected? Document any observable skin changes such as scars, wounds, fibrosis, discoloration, hyperkeratosis, papillomas, and lymph cysts. Note the presence of collateral veins, abnormal skinfolds, radiation markers, and implanted devices visible under the skin, along with any possible areas of infection. Are there areas with excessive dryness, moisture or contracture of the skin? Are there areas of lipodermatosclerosis or hemosiderin staining? Examine any notable areas more closely to assess their impact on lymphatic transport at the surface level. Will the direction and location of a scar impede normal surface drainage? Do observable surface radiation changes possibly represent deeper radiation fibrosis? Are there skin fragility issues that will have an impact on treatment? Does the observation of this tissue warrant palpation for further assessment?
Careful observation allows for safe and effective palpation of relevant tissue. Palpation will allow the therapist to gain a better sense of the affected tissue. Is the edema pitting or nonpitting? If there is pitting an objective measure must be used to document it. Is there tenderness with palpation using light touch or deeper pressure? What is the texture and temperature of the skin in the affected regions versus the unaffected regions? Is there a Stemmer sign on the fingers or toes? Are there more subtle tissue changes or is the limb fibrotic and dense? Does the affected region feel heavier on one side compared to the other? This is often the case with truncal, breast lymphedema, and with lobules of tissue on the medial thighs, back, and upper arms. The quality and location of any abnormal palpation should be documented. A body diagram may be helpful to accurately locate areas of concern. Photographs are also important, but must only be taken once written consent has been obtained and is documented in the patient’s record. Photographic technique needs to be operationally defined so that patient photographs are taken in a consistent manner. Keep the distance between the subject and the camera consistent and take photographs with similar lighting. Also ensure the patient’s privacy is protected as much as possible, keeping identifiable features out of the photo when able.
Next, measurements should be taken for limb volume, girth or other appropriate measures of the area involved (such as head, neck, breast, and genital lymphedema). It is important that anthropometric measurements are taken for comparison to a baseline height, weight, and calculated BMI. Patients going through cancer treatment often have significant changes in weight that must be accounted for throughout treatment. Circumferential measurements taken with a tape measure are standard and can be used for simple girth measurements or the numbers can be placed in volumetric programs that convert the circumferential measurements to limb volume. Commercially available volumetric programs are available for limb volumes. These programs automatically calculate the percentage reduction achieved during treatment and compare limbs, generating reports that can be part of the patient record. Other testing such as bioimpedance spectroscopy (BIS) and infrared perometry are possible measurement tools. Specific protocols should be followed to ensure standardization of any of these techniques.
ROM, muscle-strength testing, flexibility and sensation are assessed to make sure any possible deficiencies will not inhibit participation in lymphedema treatment. Posture, balance and gait must be assessed for similar reasons. If the patient will have to negotiate stairs while bandaged, this will also need to be assessed. Any deficiencies that will affect treatment outcome for the lymphedema must be addressed. Specific functional tasks should be assessed if there is any question as to the patient’s ability to perform a specific task. The clinician must anticipate the effect of any type of bandaging on the patient’s function. If bandaging creates an unsafe environment, action must be taken to remediate the situation. Patients should never be sent home with a bandage that puts them at unnecessary risk of falls or other injuries.
Patients should be given further education prior to leaving the initial evaluation. At the very least they need disease-specific information on the treatment of lymphedema. Patients should be informed about the components of CDT, the daily requirements for treatment, any costs associated with treatment (such as bandages and garments), special clothings, and appropriate shoes (required for lower extremity treatment). Meticulous skin and nail care along with risk-reduction education should be covered. The subject of garments and durable medical equipment (DME) needs to be discussed. Depending on the location, many clinics use outside vendors to provide DME services. Consent forms are required to release protected health information to companies that provide these services. The patient will need to work with these companies to ascertain his or her DME benefits for compression garments. Treatment cannot start until a clear payment source is identified for the garments and bandages. Other situations the patient should deal with because they might prevent treatment being started immediately include: obtaining further medical clearance; arranging work schedules according to the Family and Medical Leave Act (FMLA); arranging for assistance at home; obtaining reliable transportation; and obtaining necessary insurance authorizations for treatment.
The evaluation process may not be complete by the time the patient leaves the clinic. The therapist may still need time to adequately synthesize and formulate the assessment of the patient’s condition. The patient will still need clear follow-up instructions due to the complexity of the requirements for initiating treatment. It is vital that the therapist provides a written checklist or plan for the patient. This acts as a guide for upcoming treatment and continued education.
The final steps of the evaluation are to establish the prognosis with both the expected improvement and amount of time needed to obtain the improvement. A plan of care should also be determined with functional goals that have objective criteria and a temporal basis. The direct interventions include the components of CDT and any other interventions that are needed to reach the goals. A discharge plan also needs to be formulated that clearly defines when a patient will be ready for discharge to his or her self-maintenance phase of care. It is important to remember that the evaluation will often be used by insurance companies and durable medical equipment companies to determine if services will be provided. The evaluation is an opportunity to advocate for the patient to ensure he or she gets the proper care and treatment for edema. The therapist must not feel pressure to start treatment until the conditions have been met that will allow treatment to be successful. Quite often, once the patient realizes what is involved with CDT, he or she needs to make further plans prior to starting intensive treatment. With chronic lymphedema there is time to plan for treatment. Early intervention and surveillance require a different set of standards, and treatment must be timely.
Surveillance and Early Intervention
The management of cancer-related secondary lymphedema has the potential to be significantly different with the advent of pre-operative baseline assessment and early intervention management as proposed by current researchers.66 Patients scheduled to have cancer surgeries that will subject the lymphatic system to damage should be sent for pre-operative baseline assessment. Pre-operative baselines are most commonly performed prior to breast cancer surgeries, but are becoming increasingly common for melanoma, gynecological, prostate, head, neck, and certain other cancers, such as sarcomas.
The pre-operative baseline evaluation establishes a reference point prior to any treatment intervention. The evaluation assesses ROM, strength, sensation, limb volume/girth, functional activity, work status, and exercise habits. The evaluation assesses for any pre-existing deficits or impairments. It also provides patient education, activity modification, and exercises as part of a postoperative plan of care.
The early-intervention model is based on knowledge of the anatomy, physiology, and pathophysiology of the lymphatic system. The concept of a mechanical insufficiency, as outlined by Földi, is central to understanding how and why lymphedema can develop.65 This concept needs to be put into clear and concise patient-focused language, so the patient understands the importance of early detection. A simple diagram with a line representing the baseline presurgical Transport Capacity (TC) can be drawn. Then a second parallel line should be drawn somewhere below the baseline (TC) and this is called the Post-Op TC (POTC). A separate line should be drawn much further below to represent the lymphatic load (LL).
The therapist explains how the lymphatic load is normally very low, and that the difference between the LL and the TC is the functional reserve (FR). The patient gains a visual representation of the physiological process that could put him or her at risk of developing lymphedema. This serves as a basis for all risk-reduction education. Therapists can review the different components of risk reduction to show the patient how many triggers for lymphedema affect the lymphatic load, causing it to surpass the transport capacity. This information may seem too detailed, as patients are often overwhelmed at the time of cancer diagnosis. But research has demonstrated positive effects for patients receiving pre-operative information and education about lymphedema.67
Simple anatomical drawings showing superficial lymph vessels draining to their respective regional lymph nodes also help patients conceptualize the area(s) that will be at risk. Similarly, a drawing of a lymph node with its many afferent vessels and few efferent vessels can be useful for a patient undergoing sentinel lymph node biopsy. The concept that a single lymph node may have respective uptake from more than one anatomical region can be challenging for a patient. Once shown how a lymph node functions, patients gain perspective on their situation. Clear definitions and descriptions of the areas at risk are vital for the patient to have full understanding of where to look for symptoms. Previous risk-reduction and patient education for BCRL focused on arm lymphedema. However, we are well aware of the issues with truncal, chest and breast lymphedema in this population. Similarly, for other types of cancer, patients must be told from where the lymph nodes will be removed and how many will be removed. They should be shown what territories drain to those nodes and the areas at risk should be acknowledged.
The surveillance model should standardize the intervals for regular postoperative follow-up. The recent work of Stout et al. has suggested an initial follow-up after 1 month followed by surveillance assessments at 3-month intervals, at least during the first year.66 Patients should know not to delay reporting any symptoms of heaviness or tightness in the at-risk territory. It is no longer appropriate to wait until their symptoms progress. The goal of surveillance and early intervention is to pre-empt the symptoms. The therapist needs to give the patient permission to ask questions and report symptoms that used to be disregarded. The patient with subjective complaints of heaviness and fullness needs a lymphedema evaluation, even if he or she is not at one of the standardized surveillance points. Based on newer understandings of risk factors associated with development of BCRL, the onset of mild edema should never be minimized. Since most research on early intervention and surveillance is from the BCRL domain, we should not directly apply it to other cancers. Comparable research is needed to substantiate such hypotheses in other cancer-related secondary lymphedema. However, this mounting body of evidence should not be disregarded when treating other types of secondary lymphedema.
Lymphedema is dynamic in nature, especially in its early stages. Patients may be having confounding symptoms from other treatments such as chemotherapy, additional surgeries, and radiotherapy. Treatment should not be withheld during this time, especially if the patient is reporting subjective complaints of heaviness and fullness. We must not let symptoms progress to a stage where they need full decongestive treatment. These early symptoms have been shown in the literature to naturally progress to more severe symptoms without treatment.71 Health care providers worried about overtreating transient lymphedema symptoms can benefit from acknowledging a more flexible approach to treatment of secondary lymphedema in the patients at risk. Treating these early symptoms requires much less intensity. Full CDT is not required and individual components of treatment can be applied as needed. Patients are able to learn skills that will help them manage the condition over a lifetime. They may have similar symptoms in the future and will gain confidence in understanding their body and their reaction to certain activities, treatments, exercises, and compression garments.
The surveillance and early-intervention model must acknowledge deficiencies in the insurance/reimbursement system. Many countries utilize treatment only when impairment diagnoses are made.66 This is too late for the majority of patients who have known risk factors. If their insurance will only pay for garments and/or treatment once markedly visible edema is present, then we are subjecting them to a more intensive treatment model from the beginning. Using the prospective monitoring and screening model, patients and providers will need to bridge the time-sensitive gap for obtaining treatment and acquiring properly fitted garments. This will require current lymphedema programs to revamp the waiting-list concept of treating chronic lymphedema. Evidence from BCRL has shown most patients are likely to develop lymphedema in the first 3 years after treatment.64 Patients with any type of initial symptom need to see a certified lymphedema therapist in a timely fashion. Researchers have recently published various cost-analysis models that show the increased costs of treating latestage breast cancer-related lymphedema (BCRL).69,72 These studies have not included the patient-related costs for lost wages, lost leisure time, pain, psychological distress or added self-care time.66
Efforts should continue toward the active promotion of this early intervention model, even though it has not been studied exhaustively as it is based on the strong body of evidence compiled so far in the age of modern lymphedema treatment. The shift in perspective from impairment-based care to that of secondary prevention, which utilizes a prospective surveillance model to prevent common chronic disease-related sequelae,66 is vital to the advancement of the therapist role in the treatment and management of lymphedema. The work done by the National Lymphedema Network (NLN) highlights the importance of this position. In 2011, the NLN Medical Advisory Committee (MAC) issued a position paper on “Screening and Measurement for Early Detection of Breast Cancer-Related Lymphedema.”47 This concise document gives both patients with breast cancer diagnosis and those specific health care providers who treat them the guidelines for setting the prospective surveillance model as the standard of care. The strength of this document was further acknowledged when the National Accreditation Program for Breast Centers (NAPBC) issued updated guidelines in 2011 that referenced this National Lymphedema Network position paper.68
Research in Lymphedema: Issues of Measurement and Assessment of Occurrence
Quantifying and diagnosing lymphedema (LE) has been problematic to date, despite the fact that researchers have used various methods to measure the limb with LE.69 Perhaps the most common criterion for diagnosis has been a finding of 2 cm or more difference in arm circumference30 (or 200-mL difference in LV) between affected and nonaffected limbs.70 In part because of difficulties in measurement and diagnosis, the reported incidence of LE varies greatly among women treated with surgery and radiation for breast cancer. Research involving careful measures and an extended follow-up period is an important step in supplying the reliable incidence and prevalence figures needed to study and treat LE.30,43,71–74 Recent reviews of the literature have estimated the incidence of LE from 6% to 30%83 and from 6% to 62.5%.2 Petrek and Heelan75 noted that the study with the shortest follow-up (12 months) reported the lowest incidence (6%); likewise, one of the studies with the longest follow-up (11 years) reported the highest incidence (24%). This broad statistical range of findings probably reflects major breakthroughs in breast cancer treatment, including progress in breast conservation and therapeutic combinations that have led to increased survivorship;76 inconsistent criteria for defining LE;77 and small samples, retrospective analyses, and the psychometric difficulties (e.g., reliability) in assessing LE.84,86 The Missouri 60-month data from a single prospectively followed sample reflects a range of 7%–46% (6 months), 38%–82% (24 months) LE occurrence, and 43%–94% (60 months) LE occurrence, depending upon LE definition and time point (Fig. 3.28).43
There is a common misconception that LE is not a problem of the present or future due to modern procedures such as sentinel lymph node biopsy (SLNB) and breast conservation surgical approaches. However, the latest published data reveal LE occurrence to be at a significant level of concern in spite of these improved techniques.78 Clinicians and researchers report modest estimates of LE following breast cancer surgery even for SLNB-only patients.75,78 The latest American College of Surgeons Oncology Group (ACOSOG) data reveals LE incidence of 7% after SLNB only at a short follow-up at 6 months; those receiving further nodal dissection were not included in this analysis.78,79 Because this SLNB-only group with node-negative disease represents the group at lesser risk for LE and because this LE occurrence is commonly reported by clinical observation rather than objective limb measurement, there is a high probability that the condition is under-represented. Also, current protocols require further nodal dissection for node-positive disease.80 Approximately 30% of all breast cancer patients (66 000 of some 200 000 per year)81 have a positive node and therefore proceed to axillary lymph node dissection (ALND).84 Some patients will be diagnosed as node positive without SLNB, depending on the aggressiveness of the institution in staging prior to surgery.82
Fig. 3.28 Comparison of four methods for estimating lymphedema using observations from baseline to 60 months post surgery and survival analysis. (From: Armer JM, Stewart BR. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology 2010;43(3):118–127. Reproduced with permission of Lymphology.)2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree