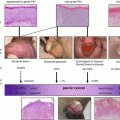
Fig. 2.1
Magnetic resonance imaging of a penile carcinoma of the glans with the proximal extent of tumor seen on the penile shaft
There is recent evidence to suggest that penile Doppler ultrasound (US) may be equivalent to MRI in the preoperative diagnostic evaluation of patients with penile SCC (Fig. 2.2) [21, 22]. In a prospective study of 200 patients presenting with a clinical diagnosis of penile SCC, penile Doppler US versus MRI accuracy in predicting primary tumor stage after surgery was 96.5 % versus 90.5 %, precision was 92.6 % versus 96 %, sensitivity was 96.9 % versus 73.8 %, and specificity was 96.2 % versus 98.5 %, respectively [23]. The authors concluded, therefore, that penile Doppler US had a statistically similar outcome in detecting tumor infiltration of the corpora cavernosa compared to MRI, and it could be used as a less expensive tool to drive surgical strategy in patient with a diagnosis of penile SCC.
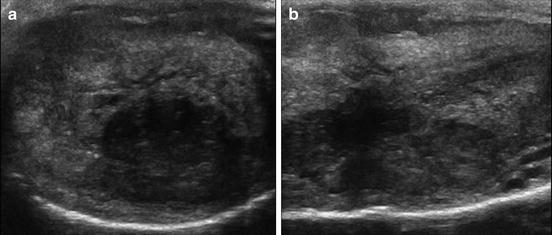

Fig. 2.2
Heterogeneous penile tumor visualized transversely (a) and longitudinally (b) on penile Doppler ultrasound
Evaluation and Management of Loco-Regional Metastatic Lymphatic Spread
Multiple different imaging modalities have been explored to more accurately predict metastatic lymphatic spread for high-risk penile tumors. The value of 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) has recently come into the spotlight in the clinical staging of penile cancer due to its increased utilization and value in other aspects of oncology [24]. Scher et al. [25] initially demonstrated the diagnostic value of 18F-FDG PET-CT in 13 patients with suspected penile cancer or suspected recurrent disease and correlated this with histopathological findings obtained at the time of biopsy or during surgery. The sensitivity and specificity for PET-CT imaging to detect malignancy in the primary penile lesion was 75 % and 75 %, respectively, but it was 80 % and 100 % for the detection of malignancy in the LNs (sensitivity: 89 % for superficial inguinal LNs, 100 % for deep and pelvic LNs) (Fig. 2.3).
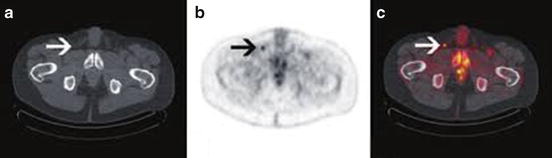

Fig. 2.3
Suspicious inguinal node detected on 18F-fluorodeoxyglucose positron emission tomography-computed tomography in a high-risk cancer. (a) CT image; (b, c) Contrast image
Leijte et al. [26] subsequently evaluated 18F-FDG PET-CT to detect occult inguinal metastasis in patients with clinically node negative (cN0) penile carcinoma. Only one of the five tumor-positive cN0 groins was correctly predicted by PET-CT although 34 of 37 negative groins were appropriately ruled out by pre-operative imaging (specificity, 92 %). This same group also evaluated the diagnostic accuracy of 18F-FDG PET-CT to detect pelvic nodal involvement in 18 patients with unilateral or bilateral tumor-positive inguinal nodes on cytological assessment. Ten of 11 tumor-positive pelvic nodal basins were correctly predicted by PET-CT scan (sensitivity 91 %) as were all 17 tumor-negative pelvic nodal basins (specificity 100 %). Four of five patients with positive distant metastasis on PET-CT had pathologically confirmed M1 disease (sensitivity 75 %). The authors, therefore, concluded that PET-CT may be useful in the routine clinical staging for inguinal node positive patients to detect further disease progression [27].
More recently, Souillac et al. [28] evaluated 22 patients with invasive SCC of the penis and negative groins (cN0) with 18F-FDG PET-CT to assess inguinal LN status. Eight patients with clinically node positive (cN+) groins were also assessed separately. Of 44 cN0 groins, PET-CT had a 75 % sensitivity and 87.5 % specificity, but it had 100 % sensitivity and 100 % specificity in cN+ groins. Schlenker et al. [29] also showed an 88.2 % sensitivity rate and a 98.1 % specificity rate for PET-CT in 70 inguinal groins (35 patients) with invasive penile carcinoma staged with this modality. All missed groin metastasis in both of the above studies were less than 1 cm in size. These results demonstrate that PET-CT may be useful in confirming inguinal LN invasion as well as detecting subclinical inguinal LN invasion in a large majority of cases although its ability to detect micro-metastasis has come into question due to higher false negative rates reported by some centers [30]. Close follow-up in these patients, therefore, is still recommended and is imperative to avoid subpar oncological outcomes.
A comprehensive systematic review and meta-analysis of the literature showed a pooled sensitivity and specificity of 80.9 % and 92.4 %, respectively, for 18F-FDG PET-CT in the accuracy of inguinal LN staging for penile SCC [31]. The pooled sensitivity was 96.4 % for cN+ patients and 56.5 % for cN0 patients. The authors, therefore, concluded that routine use of PET-CT is not justified, but patients with clinically palpable LNs may benefit due to the higher sensitivity of this technology in this subgroup of patients. Future clinical trials, however, comparing PET-CT to standard clinical assessment (i.e. physical examination) are necessary to truly elucidate the benefits that this additional imaging can provide in terms of early detection of occult metastatic disease in the groin or pelvis, minimizing patient morbidity from unnecessary treatments, and possibly improving survival-related outcomes.
Magnetic resonance imaging (MRI) and MRI-PET are additional imaging techniques that may be useful in both local and LN staging for penile cancer (Fig. 2.4). Novel magnetic resonance (MR) imaging techniques such as lymphotropic nanoparticle-enhanced MR imaging may help identify metastatic LN disease [32]. Currently, a clinical trial is being conducted and is recruiting patients in the United Kingdom to establish the effectiveness of MRI-PET compared to dynamic sentinel node biopsy (DSNB) and ultrasound-guided biopsy in detecting the presence of metastatic disease in the LNs of patients with penile cancer. If MRI-PET is effective in detecting LN involvement in patients with locally advanced penile cancer, it could potentially replace these more invasive procedures.
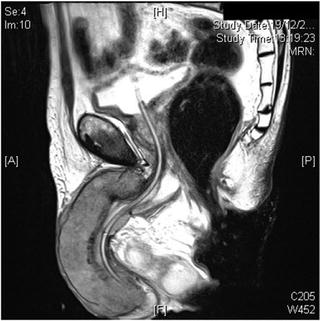

Fig. 2.4
Locally advanced penile carcinoma seen on magnetic resonance imaging
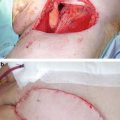
Additionally, while DSNB has traditionally been performed with radiotracer 99mTc-nanocolloid, new literature suggests the possible use of a fluorescent dye called indocyanine green (ICG) with similar effectiveness. Markuszewski et al. [33] recently reported on a small prospective study of 14 patients who underwent injection of both 99mTc-nanocolloid and ICG at the primary penile tumor site just before DSNB. Sentinel LNs (SLNs) were localized intraoperatively using the gamma-ray detection probe for radiocolloid and near-infrared fluorescence (NIRF) camera for ICG. Percutaneously, LNs were identified in all 14 patients using the gamma probe and in 10 patients using the NIRF camera. After skin incision, fluorescent nodes were observed using the NIRF camera in the remaining four patients. The intraoperative examination led to the identification of 32 total SLNs using technetium and ICG and additionally three more nodes visible only using ICG. Of the 35 SLNs, 30 were negative and 4 were positive for metastasis. Brouwer et al. [34] also reported on a hybrid radioactive and fluorescent tracer for DSNB in penile cancer as a potential replacement for blue dye (Fig. 2.5). Sixty-five patients with penile SCC underwent peritumor injection of a combination ICG-(99m)Tc-nanocolloid tracer prior to surgery followed by patent blue dye and/or NIRF imaging. Fluorescence imaging enabled visualization of 96.8 % of SLNs, while only 55.7 % were stained by blue dye (P < 0.01), suggesting a hybrid radioactive and fluorescent ICG-(99m)Tc-nanocolloid tracer can improve optical SLN detection compared with blue dye.
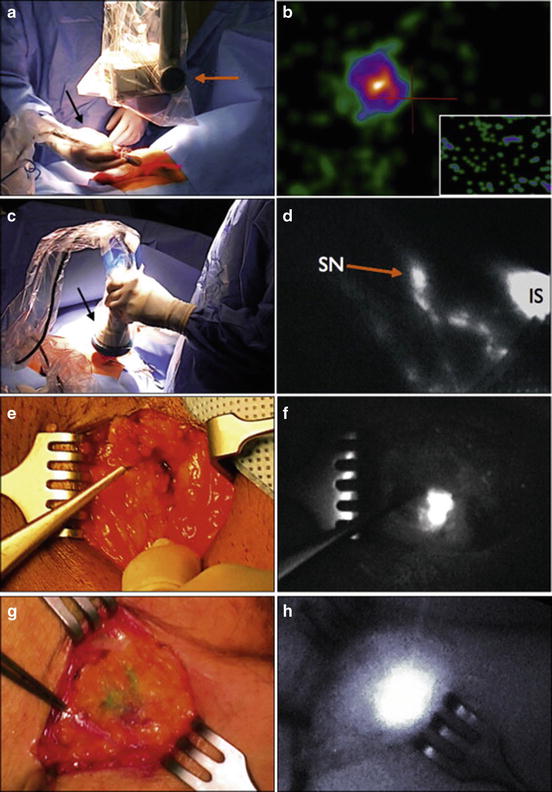

Fig. 2.5
A hybrid radioactive and fluorescent tracer technique using indocyanine green for dynamic sentinel node biopsy in penile cancer as a potential replacement for blue dye. (a, c, e, and g) Probe position at lymph node area. (b, d, f and h) Images showing sentinel node
Other future directions for novel imaging strategies in penile cancer include molecular imaging with ferrous nanoparticles or alternative nontoxic drug delivery systems. These agents can potential identify and label penile cancer cells and enhance MRI visualization of micro-metastatic disease not visible with traditional imaging [35]. Various applications using targeted iron oxide nanoparticles have been evaluated in vitro and in animal experiments for the labeling of mesenchymal stem cells and dendritic cells [36]. Future studies, however, will be needed to determine their utility in vivo in penile cancer patients.
Penile Cancer Biomarkers
Advancement in the techniques for molecular genomics has made biomarkers an increasingly important aspect of a clinician’s diagnostic and predictive tools with regard to penile tumor metastasis and disease recurrence. A list of several biomarkers studied in penile carcinoma is summarized in Table 2.1.
Table 2.1
Biomarkers in penile cancer
Biomarkers | Number of studies | Function | Prognosis |
|---|---|---|---|
p53 | 6 | Tumor suppressor gene | Expression indicated higher risk of LN metastasis, disease progression, and worse DSS |
p16INK4a | 5 | Surrogate marker for high-risk HPV infection | Positivity was associated with less tumor invasion, lower risk of disease recurrence, and possibly better survival |
Ki-67 | 4 | Marker for tumor cell proliferation in the cell cycle | Labeling correlated with higher tumor grade, advanced local tumor stage, a greater risk of nodal metastasis, and clinical disease progression |
PCNA | 2 | Marker of cell proliferation essential for replication | Expression was associated with presence of nodal metastasis |
CRP | 3 | Pro-inflammatory marker | Elevated plasma levels found more often in patients with advanced tumor stage, positive nodal disease, and worse DSS |
Cyclin D1 | 2 | Regulates progression of cells through G1-phase of the cell cycle | No clear prognostic value; implicated in tumor differentiation |
E-cadherin | 1 | Maintains cellular adhesion and signal transduction | Immunoreactivity was associated with a greater risk of LN metastasis |
MMP-2 and MMP-9 | 1 | Degrades the basement membrane of a cell | Immunoreactivity was associated with a greater risk of disease recurrence |
Fox-P3 | 1 | Oversees the development and function of regulatory T cells | Increased levels correlated to a lower inflammatory infiltrate worse OS |
ARID1A | 1 | Involved in chromatin remodeling | Higher expression was associated with a higher histologic grade |
Despite initial promising results, the evidence supporting the routine use of biomarkers in the diagnosis and management of penile cancer is still not well established enough to consider their inclusion in cancer guidelines [37]. Data are still controversial regarding the ability of biomarkers to predict the presence of occult LN metastasis. There is a need for large prospective studies to ascertain the clinical utility of biomarkers, but several candidates have been shown to be potential candidates for future investigation.
p53
Tumor protein p53 is a tumor suppressor gene that plays a role in apoptosis, genomic stability, and inhibition of angiogenesis. It can activate DNA repair proteins when DNA has sustained damage and can arrest growth by holding the cell cycle at the G1/S regulation point on DNA damage recognition. The International Cancer Genome Consortium has established that the p53 gene is the most frequently mutated gene (>50 %) in human cancer, indicating that the p53 gene plays a crucial role in preventing cancer formation [38].
Expression of p53 has been evaluated in several studies with regard to prognosis in penile carcinoma. Lopes et al. initially studied 82 patients with penile carcinoma who underwent amputation and bilateral lymphadenectomy to evaluate the prognostic value of immunohistochemical p53 staining in the primary penile tumor [39]. Immunoreactivity of p53 was studied with other clinical and pathological variables, including patient age, stage, histological grade, tumor thickness, lymphatic and venous embolization, corpora cavernosa, corpus spongiosum and urethral infiltration, and HPV status. The association of p53 with LN metastasis, survival, and risk of death was determined as the primary endpoints.
Nuclear accumulation of p53 was detected in 34 of 82 samples in the study (41.5 %) [39]. Clinical nodal stage (P = 0.045), lymphatic (P < 0.001) and venous (P = 0.04) embolization by neoplastic cells, p53 positivity (P = 0.012), and p53 grade (P = 0.004) were all significantly associated with LN metastasis. Multivariate analysis revealed that only lymphatic embolization (relative risk [RR], 9.4; 95 % CI, 2.8–31.6) and p53 positivity (RR, 4.8; 95 % CI, 1.6–14.9) were independent factors for LN metastasis. Patients with negative p53 had significantly better 5- and 10-year overall survival (OS) than those in whom tumors stained positive for p53 (64.5 % and 54.6 % vs. 30.2 % and 26.4 %, respectively; P = 0.009). When tumors were p53 positive and HPV DNA positive, OS was worse. Multivariate analysis, however, revealed that only age (RR, 2.9; 95 % CI, 1.6–5.1) and LN metastasis (RR, 3.2; 95 % CI, 1.8–5.8) were independent risk factors for death. The authors concluded, therefore, that immunoreactivity of p53 was an independent risk factor for LN metastasis, and the association of positive p53 with positive HPV DNA was related to a worse prognosis.
Martins et al. [40] reported that p53 staining exhibited correlation with penile tumor pT stage (P = 0.0005), grade (P = 0.02), lymphatic spread (P = 0.02), and CSS (P = 0.003) in 50 patients with penile SCC [40]. Multivariate analysis showed that p53 immunoreactivity was the only risk factor with prognostic significance for disease progression and CSS. Since p53 overexpression was associated with tumor progression and CSS, the authors argued that it should be evaluated in staging and therapeutic planning for patients with SCC of the penis.
Gunia et al. [41] showed p53 was an independently significant prognostic factor for CSS in penile cancer patients (hazard ratio [HR], 3.20; P = 0.041) indicating worse prognosis. Zargar-Shoshtari et al. [42] reported that positive p53 status on immunohistochemistry was associated with pN+ disease (odds ratio [OR], 4.4; 95 % CI, 1.04–18.6) [42]. Liu et al. [43] also studied risk factors for the presence of pelvic LN metastasis in penile SCC patients undergoing inguinal lymph node dissection (ILND). Primary tumor strong p53 expression was a significant predictor of pelvic LN metastasis and OS (OR, 5.997; 95 % CI, 1.62–22.3). Finally, Zhu et al. [44] reported that the expression of p53 was an independent predictor of CSS in Chinese patients with penile cancer, and in stage T1 tumors, high expression of p53 was significantly associated with metastasis and poor survival.
p16INK4a
Up to 50 % of penile SCC develops in the context of high-risk HPV infection [45]. Most of these tumors have been reported to show basaloid differentiation, and overexpression of the tumor suppressor protein p16INK4a is seen [46]. Whether HPV-triggered carcinogenesis in penile SCC has an impact on tumor aggressiveness, however, is still subject to debate with p16INK4a overexpression often used as a surrogate marker for high-risk HPV infection [8].
Steinestel et al. [47] analyzed tissue specimens from 58 patients with surgically treated penile SCC and performed p16INK4a immunohistochemistry and DNA extraction followed by HPV subtyping using a PCR-based approach. The sensitivity and specificity of p16INK4a staining to predict the presence of high-risk HPV DNA were 100 % and 57 %, respectively, and by focusing on samples with intense nuclear staining patterns for p16INK4a, specificity could be improved to 83 %. Both expression of p16INK4a and presence of high-risk HPV DNA, but not histologic grade, were inversely associated with penile SCC tumor invasion (P = 0.01, P = 0.03, and P = 0.71). However, none of these correlated with nodal involvement or distant metastasis. In contrast to pathological tumor stage, the high-risk HPV status, histologic grade, and p16INK4a positivity failed to predict CSS. These results confirmed that intense nuclear positivity for p16INK4a, rather than histologic subtype, was a good predictor for the presence of high-risk HPV DNA in penile tumors. High-risk HPV/p16INK4a positivity, independent of histological tumor grade, indicated a less aggressive local behavior, but its value as an independent prognostic indicator remains to be determined.
Bezerra et al. [48] also showed a significant association of p16INK4a overexpression and high-risk HPV status with histologic subtype (P = 0.017 and P = 0.01, respectively) and lymphovascular invasion (LVI) (P = 0.015 and P = 0.015, respectively). Regarding survival outcome analyses, neither HPV infection nor p16INK4a overexpression significantly predicted OS or CSS using Cox proportional hazards regression model.
Ferrándiz-Pulido et al. also showed that strong p16INK4a immunostaining correlated with high-risk HPV infection [49]. Both high-risk HPV-positive and p16INK4a-positive tumors showed a better OS without reaching statistical significance. The authors argued that routine use of p16INK4a staining should be incorporated in histologic evaluation of penile SCC.
Tang et al. [50] evaluated p16INK4a overexpression by immunohistochemistry for 119 consecutive patients with penile SCC50. P16INK4a overexpression was detected in 49.5 % (59 of 119) of samples. There was no significant difference between p16INK4a negative and p16INK4a positive tumors in terms of stage (P = 0.518), histological grade (P = 0.225), LVI (P = 0.388), OS (P = 0.156) or LN metastasis (P = 0.748). P16INK4a negative tumors were more likely to recur overall (P = 0.04), especially if patients had positive LNs at diagnosis (P = 0.002). These data suggest that p16INK4a/high-risk HPV status is associated with recurrence, especially in patients with positive LNs at diagnosis. Thus, patients with p16INK4a negative penile cancer, particularly those with LN metastases, may warrant closer observation after surgery.
Finally, Zargar-Shoshtari et al. [42] reported that in 57 cases of invasive penile SCC, estimated OS was insignificantly longer in p16INK4a-positive patients (median OS, 75 vs. 27 months; P = 0.27) and median CSS was not reached (P = 0.16). In a multivariable Cox proportional hazard model, when controlling for pathological nodal status and adjuvant chemotherapy, p16INK4a status was a significant predictor for improved CSS (HR, 0.36 [95 % CI, 0.13–0.99]). Only one study has shown alterations in the tumor suppressor gene p16INK4a that are associated with aggressive behavior of penile carcinomas [51].
Ki-67
Ki-67 is a nuclear matrix protein expressed in the cell cycle phases that is a marker for tumor cell proliferation [52]. Its expression can be detected by immunohistochemistry. Its prognostic value in penile carcinoma is still considered controversial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree