Normal
Prediabetes
Diabetes
Insulin not needed
Insulin needed for control
Insulin needed for survival
Type 1 DM

Type 2 DM

Other specific types

Gestational DM

Part II: Classification of DM
Though DM was recognized several centuries ago, it was HP Himsworth who first proposed that DM could be differentiated into insulin-sensitive and insulin-insensitive types in 1936 [2]. J Bornstein and RD Lawrence found insulin bioactivity in the plasma of people with maturity-onset DM but not in juvenile-onset DM [3]. SA Berson and RS Yalow conclusively demonstrated this distinction by originating insulin radioimmunoassay [4]. This formed the basis of the initial classification of DM. During that time, patients with DM were classified according to the age of onset into juvenile-onset and maturity-onset subtypes. Juvenile-onset DM is insulin-sensitive, and maturity-onset DM is insulin-insensitive. The WHO Expert Committee on Diabetes made some changes in 1980 [5]. This classification separates DM into two main categories based on insulin dependency: insulin-dependent (type 1 DM) and non-insulin-dependent (type 2 DM). This was a preferred classification as the age of DM onset did not necessarily correlate with insulin dependency. In addition, the WHO classification also included a third category termed other specific causes of DM. Causes of this category of DM include: genetic defects affecting β-cell function or insulin action, diseases of the exocrine pancreas, endocrinopathies, and drugs. There was also a fourth category termed gestational DM (GDM) that described any form of hyperglycemia first detected during pregnancy. An entity called malnutrition-related DM was introduced into the WHO classification in 1985, but was later removed due to inconclusive evidence of effects of malnutrition or protein deficiency on DM [6]. ADA proposed further changes to this classification in 1997 [7] (Table 27.2). Firstly, the terms insulin-dependent DM and non-insulin-dependent DM were eliminated, but type 1 DM and type 2 DM were retained. This was because many type 2 DM patients with progressive β-cell failure would require insulin therapy eventually and hence the term non-insulin-dependent DM might seem contradictory. Secondly, there was an inclusion of 2 subtypes of type 1 DM: type 1A, if it was immune-mediated; and type 1B, if it was idiopathic. This classification was subsequently adopted by WHO in 1999 as well, and is still in use.
Table 27.2
Etiologic classification of DM
Type 1DM |
• 1A: autoimmune |
• 1B: idiopathic |
Type 2 DM |
Other specific types of DM |
• Genetic defects of β-cell function: |
MODY types 1-6 |
Mitochondrial DNA |
• Genetic defects in insulin action: |
Type A insulin resistance |
Leprechaunism |
Lipoatrophic diabetes |
Rabson–Mendenhall syndrome |
• Disorders of the exocrine pancreas: |
Pancreatitis |
Pancreatic neoplasm |
Pancreatic surgery |
Cystic fibrosis |
Hemochromatosis |
• Endocrinopathies: |
Cushing’s syndrome |
Acromegaly |
Pheochromocytoma |
Hyperthyroidism |
Glucagonoma |
Somatostatinoma |
• Medication-induced: |
Glucocorticoids |
Nicotinic acid |
Pentamidine |
Diazoxide |
Phenytoin |
β-Adrenergic agonists |
Thiazides |
α-Interferon |
• Infections: |
Congenital rubella |
Cytomegalovirus |
• Uncommon forms of immune-mediated diabetes: |
“Stiff-man” syndrome |
Anti-insulin receptor antibodies |
• Other genetic syndromes sometimes associated with diabetes: |
Down syndrome |
Klinefelter syndrome |
Turner syndrome |
Wolfram syndrome |
Friedreich ataxia |
Huntington chorea |
Laurence–Moon–Biedl syndrome |
Myotonic dystrophy |
Prader–Willi syndrome |
Porphyria cutanea tarda |
Gestational DM |
However, assigning a type of DM to a patient frequently depends on his/her phenotype, and the circumstances present at the time of diagnosis. Sometime, the type of DM may not be evident at diagnosis. And many patients with DM do not easily fit into a single class. These will be illustrated in some of the case discussions below.
Case1
A 19-year-old student with no past medical history, was admitted for diabetic ketoacidosis (DKA), precipitated by urinary tract infection. She had been lethargic, with weight loss, polydipsia, and polyuria for 2 months. Prior to her admission, she complained of 3 days’ history of fever, dysuria, frequency, and abdominal pain. She did not have any family history of DM. She was of slim built, with a body mass index (BMI) of only 17.3 kg/m 2 .
Laboratory investigations on admission:
Total white cell count: 16.4 × 10 3 U/L (4–10)
Serum sodium: 128 mmol/L (135–145)
Serum glucose: 34.5 mmol/L (3.1–7.8)
Serum bicarbonate: 13.8 mmol/L (19–31)
Serum creatinine: 171 μmol/L (65–125)
Arterial blood pH: 7.23 (7.35–7.45)
Serum ketones: 4.6 mmol/L (<0.6)
Glycated HbA1c: 9.2 %
Glutamic acid decarboxylase (GAD) antibody: 117.4 U/mL (0–0.8)
Islet cell antibody (ICA): negative
Urine microscopy: 450 white blood cells
This is a case of a newly diagnosed type 1 DM, presenting with diabetic ketoacidosis. Type 1 DM accounts for 5–10 % of all DM cases. It is due to immune-mediated β-cell destruction, resulting in insulin deficiency, leading to hyperglycemia and lipolysis (and hence, ketoacidosis). The rate of β-cell destruction is variable, being rapid in some individuals, especially infants and children, and slower in adults. Like Case 1, type 1 DM is usually diagnosed before 30–40 years of age, most commonly during childhood or adolescence. However, type 1 DM occurs throughout life and can be misdiagnosed as type 2 DM when it presents in the middle-aged population. The lean body habitus of Case 1 is also typical of a patient with type 1 DM. Type 1 DM is usually characterized by the presence of anti-GAD, anti-islet cell, or anti-insulin antibodies, which reflect the autoimmune processes that cause β-cell destruction. Patients who have one or more of these antibodies can be subclassified as type 1A (i.e., immune-mediated) diabetes. This accounts for 80–90 % of type 1 DM. Some patients, mainly non-whites, may not have any of these autoimmune antibodies and are subclassified as type 1B (i.e., idiopathic) diabetes. Type 1 DM, especially type 1A, shows strong associations with specific haplotypes or alleles at the DQ-A and DQ-B loci of the human leukocyte antigen (HLA) complex. Despite the genetic predisposition, concordance rates for type 1 DM in identical twins are not significant [8]. Patients with type 1 DM require insulin for survival and to prevent DKA.
Case 2
A 26-year-old man was diagnosed with DM 5 years ago during a routine health screen. Both his parents developed DM in their 40s. He was overweight with a BMI of 28.3 kg/m 2 and waist circumference of 110 cm. He is currently on two oral hypoglycemic agents (metformin and sitagliptin). He does not suffer from any macrovascular or microvascular complications of DM.
Laboratory investigations during his latest outpatient clinic review:
HbA1c: 7.6 %
Triglycerides: 2.0 mmol/L (<1.7)
Low-density lipoprotein (LDL): 3.2 mmol/L (<2.6)
High-density lipoprotein (HDL): 0.9 mmol/L (1–1.6)
This is a case of a young type 2 DM. Type 2 DM is the most common type of DM, accounting for more than 90 % of the cases. This form of DM is characterized by insulin resistance, leading to relative insulin deficiency. Like Case 2, most type 2 diabetics are overweight or obese, or at least have some central adiposity. Obesity itself aggravates insulin resistance. Though type 2 DM was traditionally thought to occur only in older individuals, there is a growing incidence of type 2 DM in young adults due to the increasing prevalence of obesity in affluent countries. In contrast to type 1 DM, type 2 DM shows strong familial aggregation. DKA seldom occurs in this type of DM unless there is precipitant(s) such as infection. Type 2 diabetes is usually diagnosed late as it develops gradually and patients remain asymptomatic during the initial stages. Lifestyle modification and weight reduction decrease insulin resistance in these patients, thereby improving their glycemic control. Many of them may require OHGAs as well. The United Kingdom Prospective Diabetes Study (UKPDS) [9] had shown that despite lifestyle modification and pharmacotherapy, there is progressive loss in β-cell function, and insulin may ultimately be needed for control of these patients’ glycemic deterioration (Table 27.3, differences between type 1 and 2 DM).
Table 27.3
Differences between type 1 and type 2 DM
Type 1 DM | Type 2 DM | |
|---|---|---|
Frequency | 5–10 % of all DM | >90 % of all DM |
Etiology | Immune-mediated islet cell destruction, leading to absolute insulin deficiency; can be idiopathic | Insulin resistance with relative insulin deficiency |
Age at presentation | Usually during childhood or adolescence | Usually after middle age |
Body habitus | Usually slim | Usually overweight |
Family history of DM | Usually no | Usually yes |
Treatment | Need insulin at diagnosis | Usually treated with diet control or OHGAs, but may require insulin later |
Case 3
A 40-year-old man with generalized vitiligo, was diagnosed with DM 2 years ago when he was admitted to hospital for lower limb cellulitis. There was no family history of DM. He was of slim built with a BMI of 18.6 kg/m 2 . His physician started him on OHGAs (metformin and gliclazide) with good improvement in his glycemic control. However, despite compliance to diet and increasing doses of his OHGAs (metformin, gliclazide, glucobay), he was unable to maintain his HbA1c within 7 %. His fasting C-peptide was 0.1 nmol/L, indicating inadequate pancreatic β-cell reserve. And his anti-GAD antibodies and ICA were both positive. He was started on insulin 15 months after his presentation, with prompt improvement in his glycemic control.
Case 3’s age of presentation and the absence of insulin dependence at diagnosis seemed to suggest that he had type 2 DM. However, he had several features more typical of type 1 DM, namely, his lean body built, the lack of family history of DM, presence of GAD and ICA antibodies, as well as the rapid progression to insulin dependency. The presence of vitiligo also suggested he has a predisposition for autoimmune conditions. This is an atypical form of DM known as type 1.5 DM, latent autoimmune diabetes of adult onset (LADA), or autoimmune diabetes in adults with slowly progressive β-cell failure (ADASP). LADA is defined by three features, including: adult age at diagnosis (usually above the age of 30), the presence of diabetes-associated autoantibodies, and a delay in the progression to insulin dependency (more than 6 months, up to 10–12 years) [10]. Case 3 fulfilled all these three criteria. Studies have shown that among patients with phenotypic type 2 DM, LADA occurs in 10 % of people above 35 years old and 25 % below that age [11]. It is important to distinguish patients with LADA from type 2 DM, as treatment of choice is early insulin therapy to prevent the progression to complete β-cell failure from glucose toxicity. Sulfonylureas, which are commonly used to treat type 2 DM, have been thought to promote β-cell failure due to their stimulatory effect of insulin secretion by the pancreas, and should generally be avoided in LADA [12].
Case 4
A 36-year-old overweight Afro-American, diagnosed with type 2 DM 3 years ago (anti-GAD and ICA negative, HbA1c 8.5 %), non-compliant to his treatment with metformin and gliclazide, was admitted to the hospital with DKA. There was no evidence of infection or other precipitants for his DKA. He was switched to biphasic insulin (30 units pre-breakfast and 20 units pre-dinner) upon his discharge from hospital. However, he started experiencing hypoglycemic events at home which persisted even after he had progressively reduced the dosage of biphasic insulin to 18 units pre-breakfast and 8 units pre-dinner. His fasting C-peptide was 0.8 nmol/L, indicating presence of sufficient pancreatic reserve. He was subsequently switched back to sustained release OHGAs of metformin 1,000 mg twice daily and gliclazide 60 mg once daily. His hypoglycemic episodes resolved and his overall glycemic control improved with his HbA1c dropping to 7.2 %.
This case illustrates a seemingly typical young type 2 DM in an overweight individual. However, it is unusual for patients with type 2 DM to develop DKA, particularly in the absence of precipitants. This is an example of another atypical form of DM, known as ketosis-prone type 2 DM. It is more common in Afro-American and Hispanic patients. Hence, it is also known as Flatbush DM, in recognition of the place from where many of the original cases came from [13]. Patients with ketosis-prone type 2 DM are usually young to middle-age overweight individuals with history of acute, unprovoked episodes of DKA. Unlike type 1 DM, DKA in these patients is not due to irreversible β-cell damage, but hypothesized to be due to increased susceptibility to β-cell desensitization due to glucose toxicity [14]. Upon reversal of glucose toxicity with insulin therapy, the β-cell function partially recovers and patients should be converted to OHGAs to prevent hypoglycemia. Basal and stimulated C-peptide levels of more than 0.33 nmol/L and 0.5 nmol/L respectively shortly after presentation of DKA, as well as more than 0.5 nmol/L and 0.75 nmol/L respectively during follow-up, have been shown to be good predictors of remissions [13–15]. It is advisable to keep patients with ketosis-prone type 2 DM on OHGAs (usually low dose sulfonylurea and metformin), as normoglycemic remission periods are significantly shortened (to within 2 years) compared to if they are treated with diet control alone after discontinuation of insulin therapy [15–17].
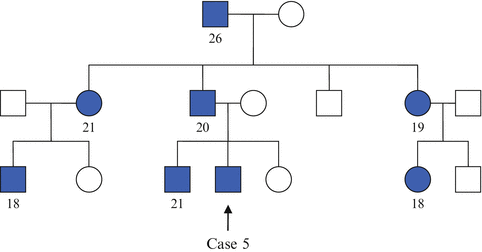
Case 5
A 19-year-old student presented with polydipsia and polyuria of few months and was diagnosed with DM. In view of his young age of presentation and lean built (his BMI is 19.8 kg/m 2 ), he was treated as for type 1 DM and was commenced on subcutaneous insulin therapy. During one of his outpatient reviews, it was realized that he has a very significant family history of young-onset DM (Table 27.4 , family tree showing affected individuals). He underwent genetic testing for maturity onset diabetes of the young (MODY) which confirmed mutation in HNF1α, one of the transcription factors that affect β-cell development and function. He was switched to gliclazide and has maintained satisfactory glycemic control since then.
Table 27.4
Family tree showing people with DM in Case 5’s family
 |
MODY is early onset autosomal dominant DM that is associated with β-cell dysfunction resulting from specific mutations in genes encoding the glucose-sensing enzyme glucokinase (GCK) or one of the transcription factors. It can be subclassified to types 1–6, according to the different gene involved. There is a seventh type called MODYx, which includes cases which the genetic mutation is still unknown (Table 27.5, subtypes of MODY). Clinical presentation (including age of onset, severity and progression of hyperglycemia) varies greatly depending on the underlying genetic mutation, but affected patients are non-insulin-dependent at the onset of the disease. In addition, they are usually of lean body habitus with no or minimal insulin resistance.
Table 27.5
Comparison of the different subtypes of MODY
MODY1 (HNF4α) | MODY2 (GCK) | MODY3 (HNF1α) | MODY4 (IPF1) | MODY5 (HNF1β) | MODY6 (ND1) | MODYx | |
|---|---|---|---|---|---|---|---|
Frequency | 5 % | 20 % | 60 % | <1 % | 5 % | <1 % | 10 % |
Chromosomal location | 20q13.12 | 7p13 | 12q24.31 | 13q12.2 | 17q12 | 2q31.3 | Not known |
Onset | Early adulthood | Birth | Early adulthood | Early adulthood
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|