21.1 Introduction
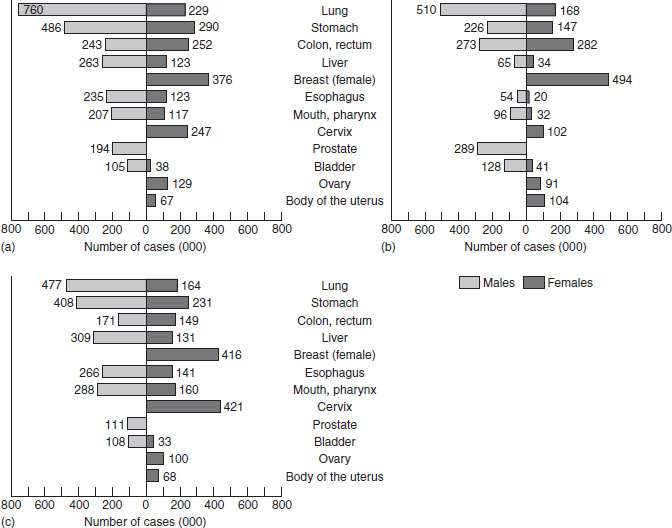
The global burden of cancer, as illustrated in Figure 21.1, is substantial. Mortality from cancer is greatest worldwide from lung cancer and stomach cancer in men, and breast cancer in women. These mortality data are reasonably good, but are still subject to misdiagnoses and underreporting in countries where cause of death is not completely reported. The incidence rates presented in Figure 21.1 (b, c) show the estimated occurrence of new cancers in developing countries and in developed countries. As true incidence data depend on complete registration of new cases of cancer, and this is done in very few countries, the incidence data are less reliable than the mortality data. The figure shows incidence per year to be greater than mortality and, contrary to expectations, that the majority of incident cancers arise from developing countries. This is a function of the disease rates and the size of the populations at risk. Survival is not addressed by these figures, and may also be impacted by diet, although this has hardly been addressed to date. Cancer survivors are particularly motivated to change their diets, but there is little available evidence to provide a foundation for good advice on what diets might best prevent cancer recurrence and enhance quality of life.
21.2 Mechanisms of effect of diet
A tumor occurs when cells within a tissue grow in an uncontrolled and progressive fashion. If the tumor is malignant, that is, if it is growing rapidly and invading adjacent normal tissue, and the cells are becoming irregular in appearance, it is called a cancer. Cancer is a genetic disease. It requires the development of rogue cells with functionally altered DNA. The sources of these alterations can be due to inheritance, damage by radiation or chemicals, and hypermethylation or hypomethylation of DNA resulting in the loss of an essential protein or the production of a harmful protein. In addition, other alterations in expression of genes or proteins can influence the risk of the disease at the promotion or progression stage. Consequently, an understanding of the ways in which nutrition and food intake can influence carcinogenesis requires knowledge of molecular biology. This includes understanding the process of cancer initiation, from procarcinogen activation to adduct formation, from DNA repair to DNA replication, from cell cycling to cell apoptosis and tumor growth.
Figure 21.1 The burden of cancer, 1996: (a) mortality, worldwide; (b) incidence, developed world; (c) incidence, developing world.
Undoubtedly our diets, as major exposures in our lives, can influence cancer occurrence and growth. Experimental models of chemically induced carcinogenesis have shown the macronutrients, various vitamins (folic acid, vitamin B12, riboflavin, retinol, β-carotene and α-tocopherol) and some minerals, including selenium, zinc, magnesium and calcium, to modulate cancer risk. Other foodborne nonnutrients such as resveratrol and lycopene also appear to influence carcinogenesis pathways. Numerous epidemiological research findings ranging from ecological comparisons of differences in risk between countries, to migrant studies of change in risk within populations, and including observational data from case–control and cohort studies as well as intervention studies, provide evidence which in part supports these and in part is contradictory to these premises from animal and cell models. The relationship between diet and cancer has only recently begun to unfold, and as the research develops, the attention on the role of diet is being drawn more away from the initiating event in cancer to the multiple roles of foods and nutrients in cell signaling, growth and apoptosis that influence tumor growth and cancer survival.
Figure 21.2 Dietary influences on carcinogenesis: initiation, promotion, progression and metastases. VEGF, vascular epithelial growth factor.
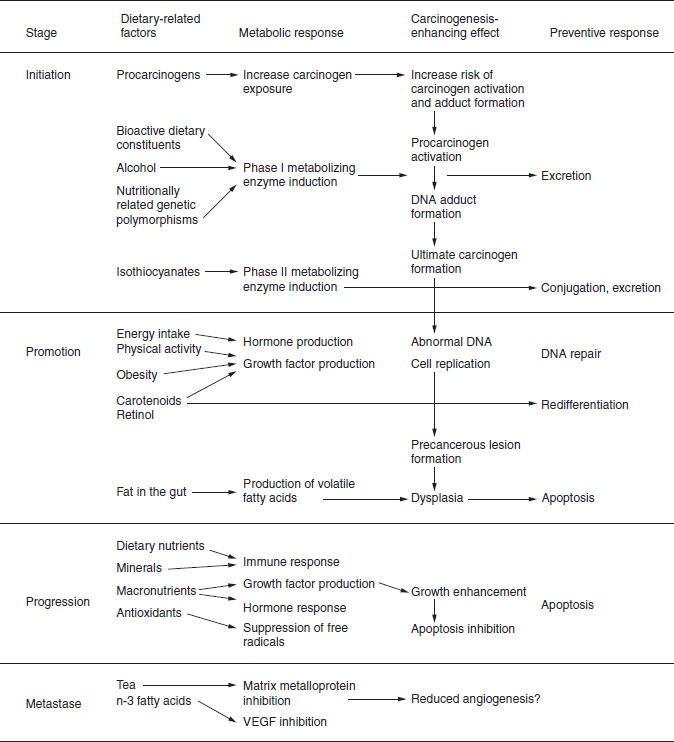
21.3 Carcinogenesis: initiation, promotion and progression to metastases
Figure 21.2 integrates the known and suspected role of diet on the carcinogenesis pathway from initiation, through promotion and progression. It illustrates where dietary components can act as the carcinogen or procarcinogen themselves, and where bioactive dietary constituents can influence the enzymes that activate procarcinogens (phase I) or the enzymes that metabolize carcinogens (phase II). Figure 21.2 simplifies the story and suggests pathways that underlie a single disease. However, cancer is not a single disease, but really a term that covers a large set of cancers that differ not only in the afflicted organ, but also in the risk factors involved. Very many dietary factors have been studied for their putative role in the causation, promotion and prevention of cancers. Many of these are summarized in Table 21.1. Readers should not presume that just because these factors have been the focus of study, they are proven carcinogens or otherwise. Much of this research is epidemiological, where true cause and effect cannot be proven. This can only be achieved by intervention studies, that for practical reasons, are rarely initated to study diet and cancer etiology. Foods contain potent mutagens and carcinogens, such as aflatoxins and heterocyclic amines, which are carried on or created within foods and are known mutagens. These activities relate to the stage of initiation of carcinogenesis. This is the part of the process during which alterations in the genetic make-up of cells are induced through the production of DNA adducts. Such adducts can result in DNA mutations or deletions if the adduct causes faulty translation or replication of the DNA, or even DNA breakage. Alterations to protooncogenes (such as ras or myc) resulting in transformations can result in unregulated cell growth or damage to tumor suppressor genes (such as p53, Rb or APC). Some nutrients, such as fatty acids, are believed to affect gene transcription directly.
This model also shows the potential of dietary factors to have an impact on hormones, growth factors and the immune system in ways that can influence promotion and progression. Promotion is the stage during which initiated cells are transformed into populations of altered cells. Tumor promoters do not affect DNA directly. Instead, they enhance cellular replication and cell growth. Pathways through which dietary constituents can enhance redifferentiation, or affect growth factor levels or cell apoptosis (programmed cell death) may also affect promotion.
Progression is the expansion of a population of initiated and promoted cancer cells to an invasive tumor mass. This is characterized by a variety of abnormalities in the DNA. This may be held in check by immune responses such as through dendritic and killer T-cells. Hormone levels can enhance tumor growth. Excessive amounts of antioxidants can theoretically inhibit apoptosis. Any dietary factors that influence the balance of cellular proliferation or mitosis to cell death can impact tumor progression. Metastases occur when these cells are no longer encapsulated and migrate to distant sites. The influence of dietary factors on metastases has been studied to some extent in animal models of clinical carcinogenesis. Among other dietary factors, tea and n-3 fatty acids appear to be modulators. The possible dietary influences on each stage are presented in Figure 21.2. As a single cancer may involve multiple initiations and the alterations of multiple genes, it should be remembered that these stages do not represent a linear progression. In fact, it is likely that all cancers result from multiple initiation and promotion cycles.
Foods can also stimulate activation of procarcinogens. The stimulatory influence of different foods on phase I enzymes, such as the cytochrome P450s, is well documented. More attention is being addressed to the multiple mechanisms by which our diets can protect against carcinogenesis by trapping singlet oxygen (carotenoids), stabilizing free radicals (as with chlorophyll) and enhancing the excretion of harmful products by stimulating conjugation.
An especially high risk of cancer results from nutrient-induced damage to protooncogenes. The activation of these genes through DNA mutation can produce growth signals to cells, resulting in uncontrolled cell growth. Once the damage is done, defensive responses include stimulation of DNA repair enzymes, a slowing of cell turnover and enhancement of apoptosis to minimize the risk of procreation of this altered DNA.
As initiation is a random, rapid and likely event, the prevention of morbidity and mortality from cancer in humans may be more strongly impacted by dietary influences that slow tumor growth. Study of the impact on growth hormones of dietary nutrients is currently actively being pursued.
21.4 Gene–nutrient interactions in carcinogenesis
Different disciplines start with different conceptual frameworks for gene–nutrient interactions. A model of levels of interaction between genes and nutrients, including the perspectives of the toxicologist (food as a carcinogen or anticarcinogen), the nutritionist (inborn errors of metabolism) and the epidemiologist (genetic polymorphisms that can determine susceptibility to nutritional influences) is presented in Box 21.1. Gene–nutrient interactions can reflect the role of genes in responding to nutrients or the role of nutrients in genetic expression. Box 21.1 emphasizes the bidimensionality of the gene–nutrient relationship. Genetic predispositions can determine the effectiveness of nutrients. Genetic susceptibility can modulate the activity and metabolism of foodborne substances. This can range from the severe and acute effects of dietary components on preconditions such as phenylketonuria to the more subtle effects of genetic polymorphisms of enzymes that influence the metabolism of procarcinogens and anticarcinogens, such as the cytochrome P450s, the N-acetyltransferases and the glutathione-S-transferases. The presence or absence of specific nutrients can precipitate the onset of disease (as with phenylketonuria) in the presence of specific genetic polymorphisms. The presence of the polymorphism, in turn, affects the rate at which a nutrient, or a foodborne toxin, is metabolized. At the other end of the spectrum, nutrients can directly affect genetic integrity and expression in ways that can enhance or inhibit carcinogenesis.
Genes determine the effects of nutrients
- Inborn errors of metabolism
- Genetic polymorphisms of metabolic enzymes (fast and slow acetylators)
Nutrients impact genetic expression
- Nutrient-induced DNA damage (aflatoxins)
- Nutrient-enhanced DNA protection (chlorophyll, antioxidants)
- Nutrient-related tumor growth or growth suppression (retinol)
- Nutrient impact on apoptosis
Table 21.1 Dietary factors that have been studied for their possible role in the cause or prevention of specific cancersa
| Cancer sites, sorted by relative frequency worldwide | Risk factors | Preventive factors |
| Lung | Possibly total fat, saturated/animal fat, cholesterol, alcohol | Fruit and vegetables, carotenoids, possibly vitamin C, vitamin E, selenium, physical activity |
| Breast | Rapid growth greater adult height, high body mass, adult weight gain, alcohol, possibly total fat, saturated/animal fat, meat | Possibly fruit and vegetables, physical activity, nonsoluble polysaccharides/fiber, carotenoids |
| Stomach | Salt, possibly starch, grilled and barbecued meat and fish | Fruit and vegetables, refrigeration, possibly carotenoids, allium compounds, whole-grain cereals, green tea |
| Cervix | Possibly fruits and vegetables, carotenoids, vitamin C, vitamin E | |
| Colon, rectum | Red meat, alcohol, possibly high body mass, greater adult height, frequent eating, sugar, total fat, saturated/animal fat, processed meat, eggs, heavily cooked meat | Vegetables, physical activity, possibly nonsoluble polysaccharides, fiber, starch, carotenoids |
| Prostate | Possibly total fat, saturated/animal fat, meat, milk and dairy products | Possibly vegetables |
| Mouth and pharynx | Alcohol, meat | Fruit and vegetables, possibly vitamin C |
| Liver | Alcohol, probably aflatoxins | Possibly vegetables |
| Ovary | Possibly fruit and vegetables | |
| Esophagus | Alcohol, possibly meat, very hot drinks, N-nitrosamines | Fruit and vegetables, possibly vitamin C, carotenoids |
| Endometrium | High body mass, possibly saturated/animal fat | Possibly fruit and vegetables |
| Bladder | Possibly coffee | Fruit and vegetables |
| Larynx | Alcohol | Fruit and vegetables |
| Pancreas | Possibly high energy intake, cholesterol, meat | Fruit and vegetables, possibly nonsoluble polysaccharide, fiber, vitamin C |
| Kidney | High body mass, possibly meat, milk and dairy products | Possibly vegetables |
| Nasopharynx | Cantonese salted fish | |
| a These dietary factors have been the subject of study, but ranking in the above table should not be taken to indicate their true role relative to one another. | ||
21.5 Epidemiological studies of diet and cancer
Cancer is not a rare disease. Although the risk of any single cancer is relatively low for an individual, the lifetime risk of developing some cancer in populations with life expectancies above 55 years is 30% or greater. The result is over 10 million new cases of cancer occurring yearly at a global level (not including skin cancer) and over 7 million cancer deaths per year. This is the result of the accumulation of risk over time. Familial risk factors for specific cancers also play an important role in risk in many individuals. Migrant studies have, however, proven that the environment plays an equally strong, if not a stronger role, in the expression of cancer within a population.
Although the relationship between diet and cancer was not widely appreciated in the 1970 and 1980s, a strong foundation of epidemiological research now exists. Research published before 1997 has been reviewed in the World Cancer Research Fund (WCRF) expert reports on “Food, Nutrition and the Prevention of Cancer: A Global Perspective”. Epidemiology of diet and cancer, as the study of human populations, rely primarily on observational data, although a few well-controlled, double-blind, randomized intervention trials on dietary constituents and cancer occurrence have been conducted. The strengths and weaknesses of various epidemiological studies in the context of the study of diet and cancer are reviewed below, along with examples of various types of nutritional epidemiological studies that have contributed to our knowledge in this field.
Ecological studies
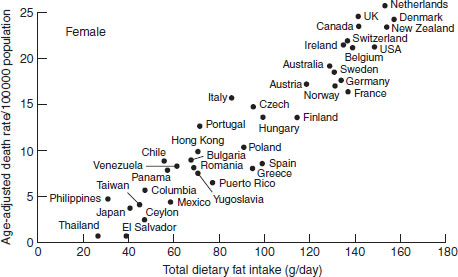
Rather than examining dietary data at the level of the individual, ecological or correlation studies examine population-wide exposures and disease rates to ascertain disease risk based on dietary intakes of groups or nations. These studies can be used to examine differences in risk across countries, but are severely limited by the potential for confounding. Ecological studies cannot examine whether the individual with low exposure is the one at higher risk. They also cannot separate out the effects of diet from those of the correlates of certain diets: drinking, smoking and consumption of other foods concurrently. However, they can be powerful indicators of strong trends across nations or over time. Examples of the illumination of possible diet and cancer relationships through ecological exploration are the strong association between dietary fat intake and breast cancer (Figure 21.3), and the close relationships between nonstarch polysaccharides and colon cancer risk (Figure 21.4). Although these associations look promising, they can be confounded by numerous other risk factors that are impossible to control for in these types of study, such as in the case of breast cancer, age at menarche, parity, age at first birth and age at menopause, all of which may vary considerably across different populations.
Figure 21.3 Ecological association between breast cancer incidence and total dietary fat intake.
Migrant studies
A major impetus to the acceptance of the fact that cancers are avoidable and influenced strongly by the environment has been provided by migrant studies that compare a set of individuals in their home countries with individuals of the same origin who have moved elsewhere. Populations who migrate to new countries tend to develop the cancer rates of the local populations, usually within one or two generations. Examples of insight gained from these studies come from the studies of Chinese men who moved to Los Angeles and Hawaii, where stomach cancer rates decreased dramatically and prostate cancer rates increased 10–15-fold, and studies of Japanese women who in migrating to Hawaii reduced their stomach cancer rates by 50% within a generation, and increased their breast cancer rates three-fold in the first generation and four-to five-fold in the second generation (Figure 21.5). This suggests that diet and other environmental factors play a larger role than genetics in the etiology of cancer.
Intervention studies of diet and cancer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree