FIGURE 1.1 Sagittal section of the upper aerodigestive tract. (Adapted from Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore, MD: Lippincott Williams & Wilkins; 2004.)
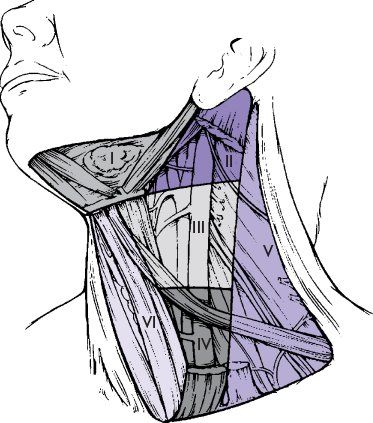
Knowledge of the lymphatic drainage of the neck assists in identification of the site of a primary tumor when a palpable lymph node is the initial presentation, and in staging metastatic spread, enabling the surgeon or radiation oncologist to plan appropriate treatment of both primary and neck disease. The patterns of lymphatic drainage divide the neck into several levels (Fig. 1.2). Level I comprises the submental or submandibular nodes, which are most often involved with lesions of the oral cavity or submandibular salivary gland. Level II (upper jugular lymph nodes) extends from the skull base to the hyoid bone, and is frequently the site of metastatic presentation of naso- or oropharyngeal primaries. Level III (middle jugular lymph nodes between the hyoid bone and the lower border of the cricoid cartilage) and level IV (lower jugular lymph nodes between the cricoid cartilage and the clavicle) are most often involved by metastases from the hypopharynx, larynx, or above. Level V is the posterior triangle including cervical nodes along cranial nerve XI, frequently involved along with level II sites in cancers of the naso- and oropharynx. Level VI is the anterior compartment from the hyoid bone to the suprasternal notch bounded on each side by the medial carotid sheath, and is an important region for spread of laryngeal and thyroid carcinomas. Level VII is the area of the superior mediastinum, and portends distant metastasis.
PRESENTATION
Symptoms and signs most often include pain and/or mass effects of tumor, involving adjacent structures, nerves, or regional lymph nodes (Table 1.1). Adult patients with any of these symptoms for more than 4 weeks should be referred to an otolaryngologist. Delay in diagnosis is common due to patient delay, repeated courses of antibiotics for otitis media or sore throat, or lack of follow-up. A persistent lateralized symptom or firm cervical mass in an elderly smoker or sexually active middle-aged adult at risk for HPV is highly suggestive of squamous cell carcinoma (Fig. 1.3). For nasopharyngeal and oropharyngeal cancers, a common presenting symptom is a neck mass, often in a node in the jugulodigastric area and/or the posterior triangle. In advanced lesions, cranial nerve abnormalities may be present. Distant metastases are uncommon at presentation, but may occur with nasopharyngeal, oropharyngeal, and hypopharyngeal cancers. The most common sites of distant metastases are lung and bone; liver and CNS involvement is less common.
FIGURE 1.2 Diagram of the neck showing levels of lymph nodes. Level I, submandibular; level II, high jugular; level III, midjugular; level IV, low jugular; level V, posterior triangle; level VI, tracheoesophageal; level VII, superior mediastinal, is not shown. (From Robbins KT, Samant S, Ronen O. Neck dissection. In: Flint PW, Haughey BH, Lund VJ, et al., eds. Cumming’s Otolaryngology Head and Neck Surgery. 5th ed. Copyright Elsevier, 2010. Used with permission.)
DIAGNOSIS, WORKUP, AND STAGING EVALUATIONS
The history should include the following:
1.Signs and symptoms as listed in Table 1.1 and above
2.Tobacco exposure (pack-years; amount chewed; and duration of habit, current or former)
3.Alcohol exposure (number of drinks per day and duration of habit)
4.Other risk factors (chewing betel nut)
5.In nonsmokers with oropharyngeal symptoms or cervical nodes, history of HPV or oral sexual practice, particularly with multiple partners
6.In nonsmokers aged 18 to 50, history or family history of anemia, Fanconi anemia, or dyskeratosis congenita
7.Cancer history of patient and family
8.Thorough review of systems

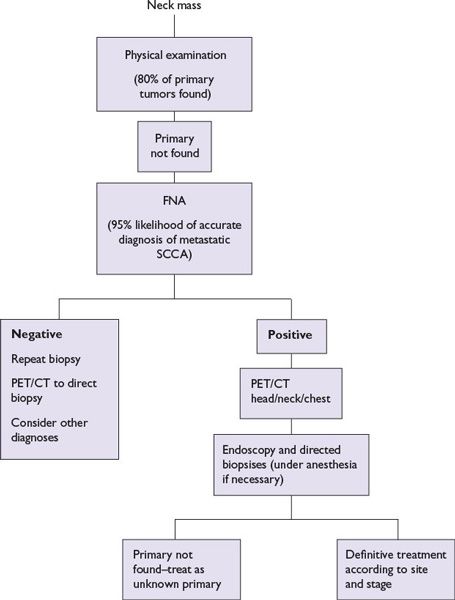
FIGURE 1.3 Evaluation of cervical adenopathy when a primary cancer of the head and neck is suspected.
The head and neck physical examination should include the following:
1.Careful inspection of the scalp, ears, nose, and mouth
2.Palpation of the neck and mouth, assessment of tongue mobility, determination of restrictions in the ability to open the mouth (trismus), and bimanual palpation of the base of the tongue and floor of the mouth
3.During examination of the nasal passages, NP, oropharynx, hypopharynx, and larynx, flexible endoscopes or mirrors as appropriate should be strongly considered for symptoms of hoarseness, sore throat, or enlarged lymph nodes not cured by a single course of antibiotics. When a neck mass with occult primary is the first presentation, the primary site can be located by clinical or flexible endoscopic examination in ~80% of cases.
4.Special attention to the examination of cranial nerves
For abnormalities identified by history, physical examination, and/or endoscopy, the following evaluations should be performed. Superficial cutaneous or oral mucosal lesions, with irregular shape, erythema, induration, ulceration, and/or friability (easy bleeding) of greater than 2-week duration warrant biopsy, as these frequently are early indicators of severe dysplasia, carcinoma in situ, or invasive malignant process. For findings or lesions involving the nose, NP, oropharynx, hypopharynx and larynx, or neck with unknown primary, computed tomography (CT) and/or magnetic resonance imaging (MRI) with contrast should first be performed to identify origin, extent, and potential vascularity of lesions. Surgical biopsy of a neck mass before endoscopy is contraindicated if a squamous cell carcinoma is suspected. Open biopsy may worsen local control, increase the rate of distant metastases, and decrease overall survival rate, possibly by spreading the disease at the time of the biopsy. An open biopsy does not provide any information additional to that obtained from fine needle aspiration (FNA), and direct laryngoscopy is still necessary for staging and treatment planning. Tissue diagnosis obtained by FNA biopsy of the node has a sensitivity and specificity approaching 99%. However, a nondiagnostic FNA or negative flexible endoscopy does not rule out the presence of tumor. Positron emission tomography (PET) scans combined with CT (PET/CT) or MRI can often localize smaller or submucosal primaries of the naso- and oropharynx that present with level II or V cervical adenopathy. Intraoperative endoscopic biopsy is then done with a secure airway under anesthesia. Bilateral tonsillectomy will sometimes reveal the source of an occult cancer, especially for HPV+ cancers. Esophagoscopy and bronchoscopy may be indicated for symptoms such as dysphagia, hoarseness, cough, or to search for occult primary.
After the diagnosis of cancer is established, the patient should be staged using physical examination, endoscopic studies, and radiologic studies, which usually include CT scan and/or MRI of the primary tumor, neck, and chest. CT scan is considered the primary imaging study for evaluation of bone involvement, regional, mediastinal, and pulmonary metastasis. MRI may complement the CT scan with greater resolution of soft tissue for primary tumor staging, and evaluation of skull base and intracranial involvement. PET/CT scans are being used more frequently to detect tumors or nodes that are not obvious on other scans and for monitoring for disease recurrence in patients with advanced locoregional disease treated with concurrent chemotherapy and radiotherapy. PET/CT scanning is indicated for staging patients with unknown primaries and for advanced head and neck cancers. A chest CT or PET/CT is indicated for all patients because of the risk of metastasis or a second lung malignancy. Body CT is not usually necessary.
Additional studies vary according to the clinical stage, symptoms, and primary site.
Specialized tests include tissue p16 immunostaining and in situ hybridization for HPV for oropharyngeal carcinoma, and tissue EBV IgA and DNA tests for nasopharyngeal carcinoma. Laboratory tests typically obtained prior to initiating therapy include complete blood counts, renal and liver function tests, serum calcium and magnesium (if platinum-based chemotherapy is to be given), baseline thyroid function tests, and pregnancy testing in females of child-bearing age. In patients with unexplained anemia, short stature, and/or micro-ophthalmia, consideration should be given to mitomycin C testing for chromosomal fragility for Fanconi anemia, as chemo- or radiotherapy is contraindicated.
Dental evaluation should be performed and any necessary extractions should be carried out 10 to 14 days prior to any planned radiation. Baseline speech, swallow, and audiometry evaluation should be performed.
Clinical staging is based on physical and endoscopic examinations and imaging tests. The staging systems of the American Joint Committee for Cancer (AJCC) or the Union Internationale Contre le Cancer (UICC) (tumor, node, metastasis [TNM], stages I to IV) are used. The AJCC classification has further subdivided the most advanced disease stages into stage IVA (moderately advanced), stage IVB (very advanced), and stage IVC (distant metastatic).
The staging of primary tumors is different for each site within the head and neck, although some common themes exist. The AJCC Cancer Staging Manual, which entered its seventh edition in 2009, should be consulted for details for each site and subsite. The T classification indicates the extent of the primary tumor. For primary tumors of the oral cavity, hypopharynx, and oropharynx, lesions up to 2 cm in diameter are T1, 2 to 4 cm are T2, and greater than 4 cm are classified as T3. For laryngeal carcinomas, limited involvement of one or more subsites are staged T1 and T2, respectively, while vocal cord immobility or pre-epiglottic space involvement with a larynx or hypopharynx primary indicates at least stage T3. Lesions with local invasion of adjacent cartilage, bone, or soft tissues indicate stage T4.
The N classification is uniform for all primary sites, except NP. Any clinical lymph node involvement indicates at least stage III. The presence of a single ipsilateral lymph node 3 cm or larger, multiple ipsilateral lymph nodes of any size, or contralateral lymph nodes of any size is classified as stage IV regardless of T stage.
The presence of distant metastasis (M1) indicates stage IVC disease. Mediastinal lymph node involvement is considered distant metastasis.
Tumor differentiation grade has not shown clear association with outcome and is not considered when staging head and neck cancers.
PROGNOSIS
The most important determinant of prognosis is stage at diagnosis. The 5-year survival for stage I patients exceeds 80% but is less than 40% in stage III and IV disease. Most patients have locally advanced disease involving one or several lymph nodes on one or both sides of the neck. The presence of a palpable lymph node in the neck generally decreases the survival rate by 50% compared to the same T stage without node involvement.
Prognoses for oropharynx cancers associated with HPV, even when locally advanced, are about 30% to 50% better than similar cancers that are not associated with HPV, but this improved outlook is reduced in smokers. A subset of patients with matted lymph nodes has been reported to have poorer prognosis.
Most relapses occur locoregionally. Distant metastases are more commonly seen later in the course of the disease or as part of relapse after successful initial treatment, and predominantly involve lung, bone, and less commonly liver. Second primary cancers after an index head and neck cancer in smokers commonly occur in the head and neck region, the lung, or the esophagus, and may represent a significant mortality risk after curative treatment of the initial head and neck cancer. Recently, there seems to have been a decline in second primary cancer incidence in patients with an index oropharyngeal cancer. This may be due to the higher incidence of HPV-related oropharyngeal cancers, as well as the likelihood that such patients are less likely to be heavy smokers.
TREATMENT
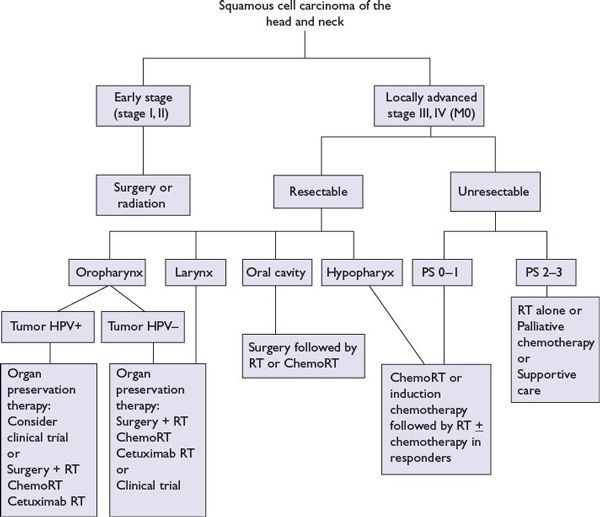
The management of patients with squamous head and neck cancer is complex (Fig. 1.4). The choice of treatment modality depends on the stage and site of disease as well as the condition of the patient. In general, either surgery or radiation is effective as single-modality therapy for patients with early-stage disease (stage I or II) for most sites. The choice of modality depends on local expertise, patient preference, and functional result. For the 60% of patients with locally advanced disease (stages III, IV, and M0), curative combined-modality therapy is indicated. These therapies could include primary surgery with adjuvant radiation (with or without concomitant chemotherapy), radiation with or without concomitant systemic therapy, and neoadjuvant chemotherapy followed by radiation with or without concomitant systemic therapy. Investigational paradigms using response to one cycle of neoadjuvant chemotherapy and biomarkers in selection of patients for chemoradiation or surgery and radiation have been reported for oropharyngeal and laryngeal cancers.
FIGURE 1.4 Treatment for head and neck squamous cell carcinomas (M0).
Patients with recurrent locoregional disease or solitary lung metastasis have benefited from surgical salvage. Some patients may be best treated with radiation, or with reirradiation to a limited field. Unresectable recurrent or distant metastatic disease is usually treated with systemic therapy with palliative intent.
Patients with squamous head and neck cancer should be evaluated before treatment is initiated by a multidisciplinary team including an otolaryngologist or head and neck surgical oncologist, radiation oncologist, medical oncologist, dentist, nutritionist, speech and swallowing pathologist, and personnel involved in rehabilitation.
Surgery
The nature of the surgical procedure is determined primarily by the size of the tumor and the structures involved. Resectability depends on the experience of the surgeon and the rehabilitation team. In general, a tumor is unresectable if the surgeon anticipates that all gross tumor cannot be removed or that local and distant control will not be achieved after surgery even with adjuvant radiation therapy. Generally, involvement of the skull base, pterygoid, prevertebral fascia and deep neck musculature, and/or the carotid artery, portends a poor outcome with surgery or other modalities. Involvement of these structures may be indicated by clinical findings such as limitation of ocular movements, tumor involving the pterygoid fossa, severe trismus, laryngeal fixation to the prevertebra, neuropathies of cranial nerves, or nodal fixation in the neck, or by CT, MRI, and PET scans.
T1 and T2 lesions of the oral cavity, oropharynx, and hypopharynx may be amenable to wide local excision with a 2-cm margin, and closed by primary or secondary intention, skin graft, or local tissue flap reconstruction. Limited carcinoma in situ and T1 and T2 lesions of the larynx may be treated by microlaryngoscopic mucosal excision or cordectomy. T2 and selected T3 cancers may be approached using one of the various external supraglottic, hemilaryngectomy or extended partial laryngectomy procedures that have been developed. Newer technologies for transoral and transnasal endoscopic surgical approaches have been recently investigated for resection of T1, T2, and selected T3 carcinomas involving the oropharynx, larynx, paranasal, and skull base region. The feasibility and outcomes for transoral laser and transoral robotic surgery (TORS) coupled with neck dissection or radiation have provided an alternative approach to chemoradiation for function sparing treatment of selected T1/T2 oropharyngeal and supraglottic primaries, and multicenter trials for comparison of these treatments have been proposed. More extensive surgeries, especially those involving the function of the tongue, oral cavity, or oropharynx, may require myocutaneous or microvascular free flaps to achieve functional reconstruction of deficits affecting mucosa, innervated muscle, and/or bone. However, as will be discussed below, with the advent of primary therapy with concurrent chemoradiotherapy for advanced T3/4 cancers of the larynx, NP, oropharynx, and hypopharynx, surgery is also being used for treatment of advanced neck disease (N2, N3) and for salvage of nonresponding or recurrent tumors of the primary site.
Cervical lymph node dissections may be elective or therapeutic. Elective neck dissections are done at the time of initial surgery in patients with necks that are clinically negative when the risk of a microscopically positive lymph node is at least 30%. Therapeutic neck dissections are done for clinically obvious masses at the time of primary surgical treatment, or persistent clinical mass, radiographic, or PET abnormalities after neoadjuvant or concurrent chemoradiotherapy. Cervical lymph node dissections are classified as radical, modified radical, or selective. The radical dissection includes removal of all lymph nodes in the neck from levels I to V (see Fig. 1.2), including removal of the internal jugular vein, spinal accessory nerve, and sternocleidomastoid muscle. Due to excessive morbidity of loss of shoulder function, this surgery is now reserved primarily for very extensive disease such as N2- or N3-stage disease with extracapsular spread involving CNXI and the sternocleidomastoid muscle. The modified radical dissection preserves one or more of the nonlymphatic structures, usually CNXI without or with the sternocleidomastoid muscle. In selective neck dissections, only certain levels of lymph nodes are removed, based on the specific lymphatic drainage from the primary site, and lack of extracapsular spread. With no palpable adenopathy, and no CT or PET scan evidence of clinical nodal involvement, nodal metastases will be present beyond the confines of an appropriate selective neck dissection less than 10% of the time. Sentinel lymph node dissection and PET scanning are currently being evaluated for use in diagnosing positive lymph nodes in patients with neck examinations that are clinically negative.
Radiation Therapy
Over the past two decades, radiation therapy has evolved to a fine art that demands a keen appreciation of both tumor biology and radiation physics. The use of CT for simulation and three-dimensional techniques for treatment planning has improved accuracy in portal design based on an improved understanding of the radiographic extent of the tumor. IMRT techniques have helped to reduce normal tissue toxicity while maintaining high doses to the target volume. The advantages of these advances have been demonstrated by an improvement in locoregional control and a decrease in normal tissue toxicity. Brachytherapy offers similar advantages when performed by experienced physicians. In addition, radiation using charged particles, such as protons or carbon ions, rather than conventional photons, has theoretical advantages for sparing of sensitive normal tissues. With the number of facilities offering charged particle radiation on the rise, direct evidence supporting the theoretical advantages is now being amassed.
Advances in diagnostic imaging have contributed to improvements in radiation therapy planning. Both PET and MRI allow better tumor delineation. Current technology allows fusion of the images from various imaging techniques on each patient so that the radiation oncologist may define the tumor and critical normal structures more accurately. While the goal of IMRT is to improve treatment planning, the goal of image-guided radiation therapy (IGRT) is to improve the accuracy of treatment delivery. IGRT involves imaging patient anatomy and adapting to patient position while the patient is positioned on the treatment machine, with the goal of targeting disease more accurately, minimizing treatment delivery variation, and more effectively sparing normal tissues. Traditionally, radiation therapy has been delivered at 1.8 to 2 Gy once daily for a total of 50 to 70 Gy with successive field reductions based on risk assessment. IMRT allows the integration of all sites into a single plan with lower-risk areas receiving lower doses per fraction while higher-risk areas receive higher doses per fraction. With this technique, gross tumor is typically administered daily doses higher than 2.1 Gy.
Altered fractionation schemes have had mixed success. These include hyperfractionation (1.2 to 1.5 Gy twice or thrice daily) and the concomitant boost technique (1.8 Gy in the morning to the entire field followed by 1.5 Gy in the evening to a smaller field encompassing high-risk disease). With either schedule, it is essential to maintain 4 to 6 hours between fractions to allow normal tissue repair. Although altered fractionation improves outcome, this is offset by an increase in acute toxicity without increase in long-term complications. The integration of chemotherapy with altered fraction schedules is under investigation. However, preliminary results of a phase III trial (RTOG 0129) comparing standard fractionated to accelerated fractionated chemoradiation showed no difference in outcome or late toxicity between the groups. Early-stage (T1, T2, N0) disease responds well to single-modality treatment with either surgery or radiation therapy. Radiation therapy allows organ preservation—as evidenced by its role in the management of early-stage cancers of the glottic larynx and pharynx. However, more advanced disease (generally, stage III and IV) requires the integration of radiation therapy with other modalities.
Toxicity of Radiation
With the advances in radiation treatment planning and delivery, toxicities associated with radiation are less than they were two decades ago. Common severe acute radiation toxicity includes dermatitis, mucositis, loss of taste, xerostomia, dysphagia, and hair loss. Decreased hearing is uncommon. Dental evaluation and necessary extractions should be performed before radiation because dental extractions in a radiated mandible can lead to osteonecrosis. Dentulous patients should be given prophylactic fluoride. Patients receiving radiation are at high risk for tooth decay due to the xerostomia caused by injury to the salivary glands as well as mucosal damage. Radioprotectors such as amifostine and pilocarpine have not demonstrated a consistent ability to decrease xerostomia. IMRT techniques enabling the reduction of dose to the parotid glands have had more success. Similarly, permanent swallowing dysfunction can be avoided by decreasing the dose to the pharyngeal musculature. Prophylactic, pretreatment, and posttreatment evaluations by a speech therapist also help in preventing and alleviating dysphagia in these patients.
Concomitant Chemoradiation
Radiation with concomitant chemotherapy is used with the intent of organ preservation when surgery would result in the compromise of voice and swallowing functions. It is also used in patients who have stage IVB disease in attempt to cure a patient for whom surgery is not considered a good option (patient not medically fit for surgery or is disease is considered “unresectable”) or IVC disease when, although palliative, local control is desired. Studies have evaluated the use of chemotherapy administered before radiation or surgery (i.e., neoadjuvant or induction chemotherapy), instead of surgery (i.e., concomitant chemotherapy and radiation) or after surgery (i.e., adjuvant chemotherapy and radiation). The rationale for concomitant chemoradiation is based on experimental evidence of synergism between chemotherapy and radiation that is theoretically mediated by interference by chemotherapy with multiple intracellular radiation-induced stress-response pathways involved in apoptosis, proliferation, and DNA repair. The finding that certain chemotherapeutic agents (e.g., cisplatin, 5-fluorouracil [5-FU], taxanes, and hydroxyurea) can induce radiosensitivity and increase log cell kill from radiation supports this treatment strategy. Cisplatin, the most extensively evaluated drug in large randomized trials, has the advantage of not having mucositis as toxicity; although as a radiation enhancer, it does increase radiation-induced mucositis.
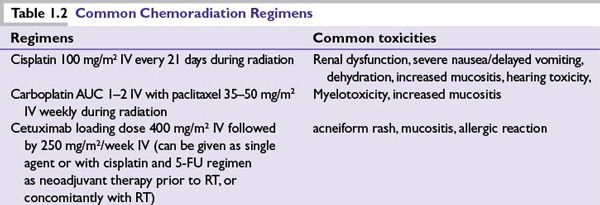
Radiation administered concurrently with chemotherapy or the anti-EGFR antibody cetuximab has been shown to improve survival in patients with advanced head and neck cancers (Table 1.2). Randomized clinical trials and meta-analyses show that for locally advanced head and neck squamous cell carcinoma, concomitant chemoradiation (with cisplatin) produces a small but significant survival advantage of about 8% at 5 years compared to radiation therapy alone. The U.S. Intergroup compared concomitant cisplatin and radiation to split-course radiation with cisplatin and 5-FU to standard radiation alone in patients with unresectable head and neck squamous cancer and showed that concurrent cisplatin at 100 mg/m2 every 21 days with daily radiation (5 days per week) significantly improved survival rates. Administration of concurrent cisplatin with radiation is also associated with higher rates of larynx preservation in locally advanced larynx cancer, compared to radiation alone. More frequent dosing of cisplatin (e.g., weekly or daily) is postulated to increase sensitization, and is an area of active investigation. A randomized trial of neoadjuvant cisplatin and 5-FU followed by radiation versus concurrent cisplatin and 5-FU with radiation in patients with unresectable head and neck cancer showed similar survival rates but improved locoregional control for the concomitant arm. Results have been presented in abstract form for patients with stage II to IV resectable cancers, comparing a taxane-based triplet neoadjuvant regimen followed by radiation and concomitant weekly carboplatin (or accelerated boost radiation with weekly Docetaxel) to concomitant accelerated boost radiation with cisplatin given every 21 days. This phase III trial showed no difference in 3-year survival, though poor accrual caused early stopping. A second phase II trial, comparing radiation given concurrent with docetaxel, 5FU and hydroxyurea, both given every other week with or without neoadjuvant taxane-based triplet chemotherapy showed better disease-free survival but similar overall survival for the neoadjuvant arm. Consequently, concomitant platinum-based chemoradiation may be considered for patients with unresectable advanced head and neck cancer with good performance status.
Concomitant chemoradiation regimens using taxanes with either 5-FU or cisplatin show promising results as do regimens containing 5-FU and hydroxyurea with concomitant twice-daily radiation, with both chemotherapy and radiation administered together every other week. Agents that inhibit EGFR signaling have been evaluated as radiation enhancers in head and neck squamous cancer. More than 90% of head and neck squamous cancers express EGFR, and increased expression has been correlated with poorer survival rates after radiation therapy. The EGFR inhibitory monoclonal antibody cetuximab has been shown to result in an enhancement of response and survival over radiation alone, although more than 50% of the trial participants had oropharyngeal primary tumors, a type previously associated with greater responsiveness to radiation. In contrast to trials comparing radiotherapy with or without chemotherapy, there was no reduction in distant metastases in the cetuximab arm. Clinical studies are ongoing with combinations of EGFR inhibitors, with radiation and with standard chemotherapy agents. Preliminary reports of RTOG 0522 showed no progression-free or overall survival benefit with the addition of cetuximab to standard cisplatin-based chemoradiation, although mature results are awaited. Recent studies suggest that additional molecular alterations, in addition to EGFR, are likely to be important for response, such as nuclear factor-kappaB (NFκB), signal transduction and transcription-3 (STAT-3), and inactivation or mutation of tumor suppressor p53, mutation or overexpression of MET, as well as epithelial-to-mesenchymal transition. Agents targeting these pathways individually, such as bortezomib, quinacrine (NF-κB, p53), and STAT decoy, have shown limited activity.
After chemoradiation in patients with N2, N3, or multiple nodes at diagnosis, elective lymph node dissection may be carried out when complete response is obtained at the primary site, especially when there is less than complete nodal response to chemoradiation. N2 or greater nodes often (about 20%) harbor tumor even if a clinically complete response is obtained in the neck with chemoradiation. Surgical salvage may be attempted if complete control is not achieved at the primary or locoregional site. Major complications with surgical salvage are found in about 52% of patients previously treated with organ-preserving regimens.
Postoperative/Adjuvant Therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree