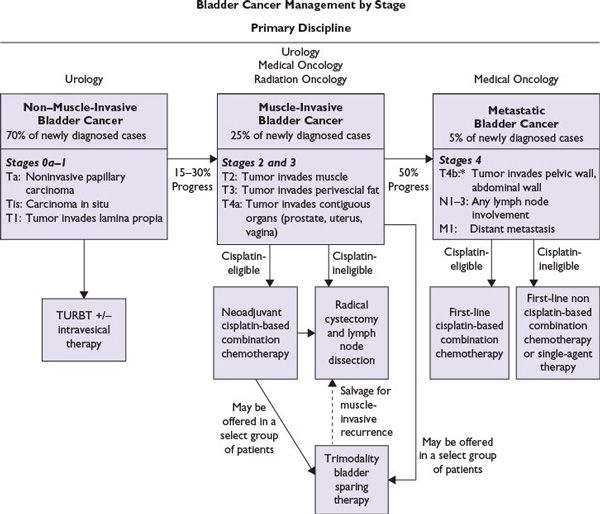
FIGURE 15.1 Treatment of bladder cancer by stage: The management of bladder cancer differs significantly depending on stage. This algorithm depicts the treatment of non–muscle-invasive, muscle-invasive and metastatic bladder cancer. *T4b, if tumor responds to systemic chemotherapy, consolidation with a radical cystectomy may be considered.
PROGNOSIS
■The major prognostic factors are tumor stage at the time of diagnosis and degree of tumor differentiation.
■Five-year survival rates for patients with non–muscle-invasive, muscle-invasive, and metastatic bladder cancer are 95%, 50%, and 6%, respectively. Median OS for non–muscle-invasive bladder cancer is 10 years, with a natural history characterized by recurrence of non–muscle-invasive tumor or progression to muscle-invasive disease. Non–muscle-invasive tumors recur in 60% to 70% of cases; about one-third of these progress to a higher stage or grade. Significant variability in OS occurs in patients with metastatic urothelial cancer undergoing first-line treatment with chemotherapy. In order to better predict OS in these patients, Memorial Sloan-Kettering Cancer Center (MSKCC) developed a prognostic model using two pretreatment risk factors: Karnorfsky performance status (KPS) less than 80% or the presence of visceral metastases (liver, lung, or bone). Based on the MSKCC prognostic risk group model, patients with no risk factors had a median survival of 33 months; 1 risk factor, 13.4 months; and 2 risk factors, 9.3 months (P = 0.0001). There is now a new model modified by MSKCC to include four pretreatment variable including visceral metastases, performance status, albumin, and hemoglobin. This new four-variable prognostic model for patients with metastatic urothelial carcinoma has a statistically significant superiority for predicting OS in patients with metastatic disease than the former two-variable model. The prognostic model can predict the survival probabilities at 1-, 2-, and 5-year and median OS in patients with metastatic urothelial carcinoma.
TREATMENT
Figure 15.1 shows an algorithm for treatment of bladder cancer.
Non–Muscle-Invasive Bladder Cancer
■TURBT remains the cornerstone of treatment for non–muscle-invasive disease, i.e., Ta, T1, and Tis bladder cancers. A second TURBT may be performed for high-grade tumors. In addition to observation after TURBT, intravesical therapy may be used. Close follow-up is recommended for high-risk tumors (high-grade Ta, CIS, and high-grade T1) with urine cytology and cystoscopy every 3 to 6 months for the first 2 years and longer subsequent follow-up intervals after 2 years as appropriate.
■Intravesical therapy is primarily used as an adjunct or prophylaxis after TURBT to lower the incidence of disease recurrence and/or progression. Intravesical chemotherapy is used for low-risk disease (low-grade Ta and T1) and intravesical bacillus Calmette-Guerin (BCG) therapy is recommended for high-risk disease (high-grade Ta, CIS, and high-grade T1). Intravesical chemotherapy: Chemotherapeutic agents used for intravesical instillation include thiotepa, doxorubicin, epirubicin, and mitomycin C. Data suggest that currently available intravesical chemotherapeutic agents are equally effective but differ in toxicity. Although no standardized dosing or scheduling has been established as the optimum delivery for intravesical chemotherapy, a meta-analysis showed one dose of cytotoxic chemotherapy reduced the risk of recurrence by 39%. Patients with low-grade solitary papillary tumors particularly benefited. Thus, in addition to observation after TURBT, an option for a low-grade, clinical stage Ta lesion would be administration of a single dose of intravesical chemotherapy within 24 hours of TURBT. Mitomycin C is the chemotherapy most often used. Immunotherapy with BCG has shown statistically significant clinical benefits, including induction of complete response (CR) in CIS (70% to 75%) and reduction of rates of recurrence in high-grade Ta of T1 (20% to 57%), but has shown no consistent reduction in tumor progression. Maintenance BCG has been shown to reduce recurrence, but optimal dose scheduling and duration have not been determined. A second induction of BCG therapy may be given, at 3-month follow-up, to recurrent/persistent tumors that responded to initial intravesical therapy. No more than two consecutive induction courses should be given. If disease recurs after two consecutive BCG inductions, then cystectomy is advised. Intravesical valrubicin is FDA approved for BCG-refractory patients who refuse or are intolerant of cystectomy. Other agents used in this population include gemcitabine and cotreatment with BCG and interferon α-2b.
■Early radical cystectomy indications include BCG-refractory CIS or high-grade lesions that recur after BCG immunotherapy. High-grade T1 and CIS lesions have a propensity to progress and even metastasize. Nonsurgical candidates may pursue a clinical trial with alternative therapies including chemoradiation.
Muscle-Invasive Bladder Cancer
■Radical cystectomy with bilateral pelvic lymph node dissection and distal ureterectomy is the standard therapy for muscle-invasive bladder cancer. In men, the surgery involves removal of the prostate gland, seminal vesicles, and proximal urethra. In women, it involves removal of the urethra, uterus, fallopian tubes, anterior vaginal wall and surrounding fascia.
■Trimodality therapy with chemotherapy, radiotherapy, and TURBT: Definitive chemoradiation is an alternative to radical cystectomy with the goal of bladder preservation. The two most common approaches include protocols developed at Mass General Hospital (MGH), the University of Paris, and the University of Erlangen. The MGH and University of Paris protocol patients undergo complete TURBT followed by an induction dose of chemoradiation therapy; the patients are then assessed for response. Only patients who achieve a CR then undergo consolidative chemoradiotherapy for bladder preservation, whereas patients who do not achieve a CR are referred for radical cystectomy with curative intent. In the University of Erlangen protocol, patients receive upfront full-dose chemoradiotherapy and then are evaluated for therapeutic response; patients who do not achieve a CR then undergo radical cystectomy. Cisplatin is the most common radiosensitizer used in trimodality therapy; however, cisplatin is not ideal, since many bladder cancer patients who are referred for radiotherapy have impaired renal function or poor performance status. The combination of fluorouracil and mitomycin C is good alternative for patients who are not cisplatin candidates. Patients with multifocal disease, CIS, or hydronephrosis are not ideal candidates for definitive treatments with trimodality therapy. Combined chemotherapy with radiotherapy significantly improved locoregional control of bladder cancer, as compared with radiotherapy alone, without significant increase in adverse events.
■Neoadjuvant cisplatin-based chemotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






