Fig. 10.1
McClemont Cone Effect : When a person is lying or sitting, muscle, fat, and skin are sandwiched between bone and the external surface. Prolonged compression of capillaries that supply these tissues causes ischemic compromise, particularly in tissues closest to bone. Pressure transmitted to these deeper layers of tissue can be up to five times that on the epidermis
The most susceptible areas are over bony prominences; the most susceptible persons are those with limited or dependent mobility or sensory impairment [13]. Although current hospital practice dictates turning the body every 2 h to offload pressure on high-risk body parts, in truth, there is no clear minimum amount of pressure or time necessary to induce cell damage, and the practice of turning the body every 2 h is more convention than evidence-based therapy and prevention [14].
Shear forces are potent factors in the development of advanced wounds in home-based settings. Shear forces cause stretching and disruption of subcutaneous tissues and capillaries, making them far more vulnerable to damage by pressure [15]. Typical shear stresses in homebound persons include those generated on the sacrum when a person is shifted upward in bed or glides downward gradually from a partially upright position of 30–45°, and on the hip when the person is turned to the side by dragging the body along the greater trochanter. Shear forces accelerate the rate of ulcer manifestation and progression by several orders of magnitude over pressure alone. Older persons with more fragile and inelastic integument due to loss of collagen anchoring proteins and slower rates of tissue repair are more susceptible to injury from shear forces [16]. Minimizing or eliminating shear strain is a crucial primary and secondary prevention strategy, as this force more than pressure alone has been implicated in rapid tissue breakdown and ulceration [10].
10.3.2 Ulcer Stages and Categories
Stage/Category I ulcers emerge without frank denudation or ulceration of skin that is red and nonblanchable [8]. In persons with darker skin, these areas are hyperpigmented and the distinction as a pressure ulcer is often the relative warmth or tenderness to touch compared to the surrounding areas. Stage I pressure ulcers are complex wounds to categorize anatomically; though the skin is intact, the degree of damage to deeper tissues may be substantial. If the cause is friction, the damage to deeper tissues may be limited and thus the true thickness of tissue involvement is limited to the epidermis. The NPUAP and EPUAP caution against the classification of hyperemia exclusively due to friction a Stage I ulcer; however, many experts agree that distinguishing superficial hyperemia due to friction is difficult in clinical practice and perhaps underestimates the potent role that friction plays in the culmination of decubitus ulcers [8, 14]. Given the high likelihood of substantial deep tissue injury when pressure is the cause, Stage I ulcers should never be taken lightly; rather they should be seen not simply as at-risk skin involvement but serious deep tissue injury [6, 14, 17] (Fig. 10.2).
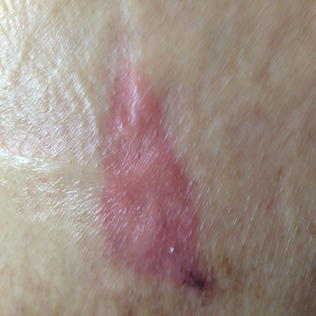

Fig. 10.2
Stage/category I pressure ulcer of the buttock
Stage/Category II ulcers are partial thickness wounds involving the epidermis and dermis [8]. They usually present as visible denudation of the epidermis and often have depth on the order of millimeters. They may also present as fluid-filled blisters. Although Stage II ulcers may be the result of pressure, they are more frequently the outcome of substantial friction over bony or nonbony areas. They may be promulgated by moisture from urine or feces, heavy exudate or sweat that separates the bonds between keratinocytes in the stratum corneum, a phenomenon called maceration [14, 18]. Most Stage II ulcers do not progress to Stage III or IV wounds [6, 17].
Stage/Category III ulcers are shear force or pressure injury wounds. They appear as full thickness skin loss involving damage or necrosis of subcutaneous fat that may VISIBLY extend down to, but not through, underlying fascia, with the ulcer presenting to the naked eye as a deep crater [8, 14]. Classically, these are beefy red craters with depth. Depth, however, can vary based on the anatomic location. In areas in which there is little to no subcutaneous tissue, such as the ankle or scalp, a Stage III ulcer may be quite shallow; in contrast, in areas such as the buttock that have a substantial amount of fat, the depth can be substantial. Stage III ulcers may also extend underneath the dermal and subcutaneous layers of adjacent tissue, a phenomenon known as “undermining” or “tunneling.” Deeper tissues such as muscle, tendon or bone, although likely affected, are not visible to the naked eye (Figs. 10.3 and 10.4).
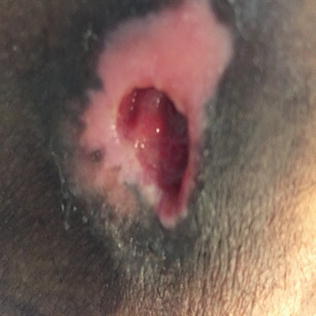
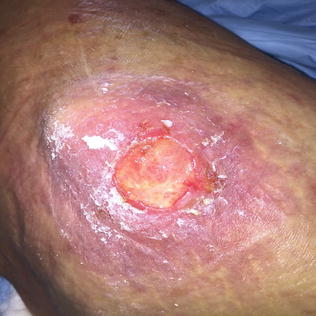

Fig. 10.3
Stage/category III ulcer of hip

Fig. 10.4
Stage/category III ulcer with superficial slough and surrounding cellulitis
Stage/Category IV ulcers are true pressure injury wounds. Like Stage III ulcers, they present with full thickness tissue loss; in contrast, however, deep tissue layers such as muscle, tendon, ligaments, or bone are visible. All other characteristics of Stage III ulcers apply to Stage IV ulcers including widely variable depth depending on the anatomic location and transgression to adjacent tissues through undermining and tunneling (Figs. 10.5 and 10.6).
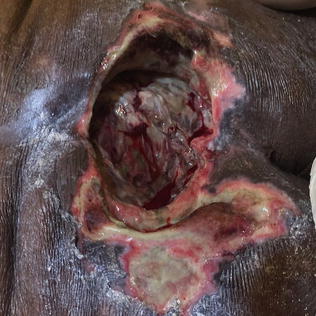
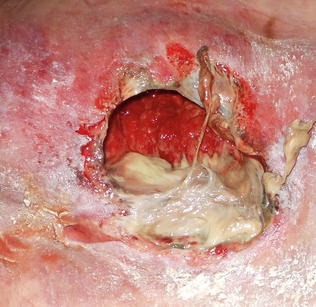

Fig. 10.5
Stage/category IV ulcer of sacrum with extensive necrosis

Fig. 10.6
Stage/category IV ulcer with dense slough and maceration of the periwound
Unstageable ulcers present as full thickness skin loss but the true depth of the ulcer is obstructed by necrotic tissue in the form of slough or eschar. Slough is characteristically yellow, tan, gray green, or brown and may be dense and adherent or soft and viscous. Eschar is tan, brown, or black and can also be dry and adherent or soft and pliable. It is important to recognize that though these necrotic elements may be located on the edge of Stage III and IV wound beds, wounds described as “unstageable” have such elements at critical points which obscure the fullest tissue depth of the wound. Until the slough or eschar is removed from the base of the wound bed, the actual depth of tissue involvement cannot be determined. This practice is generally contraindicated, however, when heel ulcers are the sites of injury. The development of eschar on the heel is considered protective, so dead tissue should not be removed if it is dry (Figs. 10.7 and 10.8).
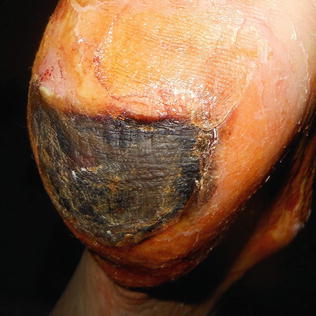
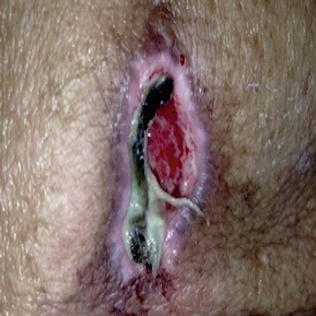

Fig. 10.7
Unstageable heel ulcer with eschar

Fig. 10.8
Unstageable sacral ulcer with extensive peripheral eschar and slough
Suspected Deep Tissue Injury (DTI) characteristically presents as either a blood-filled blister or ecchymosis with purple or maroon colored intact skin in light-pigmented persons and brawny intact skin in darker-pigmented persons. Affected areas can feel edematous or boggy and are often warmer and tender compared to adjacent skin. DTI pressure ulcers are classically located over bony prominences with highest prevalence at the heel and lesser but substantial prevalence at the sacrum and buttocks [19] (Fig. 10.9).
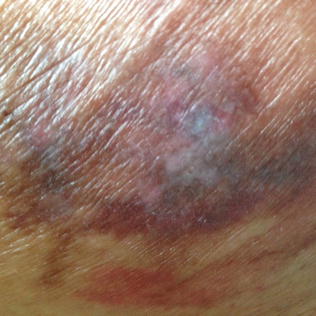

Fig. 10.9
Suspected deep tissue injury (sDTI) of the buttock
10.3.3 Effective Turning and Repositioning Techniques in the Home-Based Setting
High-risk skin areas in bedbound individuals should be inspected thoroughly at every home-based medical care visit. These include, in greatest incidence, the sacrum, ischium, heels and ankles, greater trochanters, and scapulae [20] and with lesser incidence the back, elbows, ear lobes, and scalp. Areas of vulnerability, however, can vary in persons with paralysis, kyphosis, and other anatomic abnormalities.
Proper repositioning is important for home-based medical care providers to understand; providers should instruct caregivers and interdisciplinary personnel on accepted methods for high-risk patients and for those with existing ulcers [21]. All providers should keep in mind, however, that optimal repositioning and repositioning schedules are controversial and little study has been devoted to the potential significance of small frequent weight shifts that may ultimately prove more practical and potent in home-based settings [22]. Regardless, we recommend the coupling of repositioning and frequent daily skin inspection as integral to preserving skin integrity in immobile patients with execution of the following repositioning and pressure-offloading techniques whenever possible. The following are suggested methods of positioning and repositioning to minimize friction, pressure, and shear force:
Use bed linens as a shield and sling to turn and lift immobile persons in bed, rather than drag the body directly over surfaces.
Allow partially mobile persons to move themselves when possible; use assistive devices such as a trapeze to assist patients in mobilizing themselves. Small movements at regular intervals are advised and caregivers may prompt for regular self-repositioning.
When recumbent, place persons on their sides at a 30° tilt; avoid placing immobile persons directly on their sides to limit pressure on the greater trochanter and lateral malleolus of the ankle [23]. This is an awkward position for most people to maintain so the use of pillows is essential.
Frequently turn and reposition lying patients every 2 h and seated patients every 15 min. The repositioning interval can be prolonged up to 4 h for recumbent positions and 1 h for seated persons when using pressure-reducing surfaces although this practice has not been validated [22].
With the exception of eating and 30-min postprandial periods, do not leave immobile persons in an upright position more than 30° unless they are on a low air-loss mattress. Upright angles greater than 30° have been associated with higher sacral pressures when persons are on standard mattresses.
Remain cautious about points of pressure and when possible keep bony prominences offloaded; for instance, never allow heels to remain static on a supine surface. Rather, elevate with pillows at placed just under the calves to keep heels off the bed surface. Pay attention to pressure points on bony surfaces when patients are placed in wheelchairs or transfer slings.
Train and monitor caregivers regularly on effective repositioning and offloading. These techniques are not intuitive.
10.3.4 Ulcer Risk Scales and Utility in Clinical Practice
Several widely accepted scales are used in hospital based and nursing home settings to define the severity of internal and external risk factors that are most likely to lead to pressure ulcers. Though these scales have not been studied in home-based settings, their scoring breakdown is useful in determining and mitigating risk more holistically.
Decubitus ulcer risk scoring is a key component of the Outcome and ASsessment Information Set (OASIS) [24], which is used by Medicare-certified home health agencies (CHHAs) and must be conducted for each patient at defined intervals including on admission. The Braden Scale [25], a commonly used tool in the US, emphasizes risk as a mix of internal (patient specific) and external (environmental and physical) factors.
Because current evidence has shown that the use of risk assessment tools is not more effective than clinical judgment alone in preventing ulcers, home-based medical providers rarely use such time-intensive scales [26–28]. Nonetheless, it is important to think of these scales as a framework on which to determine risk and base prevention and therapy, as well as a common language for the interdisciplinary care team. We summarize the essentials here as a general inventory of risk factors. In addition, we have supplemented risk factors with practical considerations beyond what is captured in accepted scales.
10.3.4.1 Patient Characteristics Influencing Pressure Ulcer Development
Physiologic Age : Advancing age with increased comorbid conditions that enhance the likelihood of incontinence, skin breakdown, and reduced capability of repair all contribute substantially to intrinsic risk.
Comorbid conditions : Diseases such as cardiopulmonary disease, atherosclerotic disease, or diabetes, which impair tissue oxygenation increase the risk of tissue ischemia and thus pressure ulcer formation. Diseases such as dementia promulgate other risk factors such as immobility, sensory impairment, nutrition, and incontinence; dementia of all types poses the greatest risk for pressure ulcers.
Degree of immobility : Poor or limited ability to make small adjustments in movement necessary to offset pressure-related ischemia is critical to the development of wounds. Highly immobile patients such as those with paraplegia, severe degenerative joint disease, or advanced dementia make them far more likely to experience high shear forces when mobilized and develop pressure wounds if not repositioned frequently, particularly if they are unable to make the small bodily adjustments that are critical to avoiding nocturnal ischemia in mobile persons.
Cognition : Persons who suffer from impaired cognition or communication may lack the ability to move or direct their caregivers on turning and repositioning, particularly when in discomfort from prolonged periods of pressure. Furthermore, such persons may be incontinent and thus may be prone to urinary or fecal dermatitis.
Sensory impairment : Those with impaired sensation such as those with spinal cord injuries, peripheral vascular disease, or scarred integument from previous deep tissue ulcers are unable to feel the painful stimuli from pressure-related ischemia and therefore unlikely to spontaneously move to relieve pressure.
Nutritional state : A compromised nutritional state which often accompanies advancing age, frailty, and multiple comorbid conditions, particularly dementia, are clear risk factors for pressure ulcer formation. For one, as the patient loses weight, contact points of bony prominences are more pronounced and susceptible to pressure and friction injury. Additionally, the reduction in protein due to reduced intake or increased catabolism, as well as other nutrients such as vitamin C and zinc causes tissues to lose reparative capability [29]. Substantial controversy exists on whether nutritional supplementation with either protein or vitamin C and zinc actually improves outcomes. Typically, in persons with Stage III and IV ulcers, we advise a high protein diet (up to 1.5–2 g/ kg/day) with the addition of a daily multivitamin that contains vitamin C and zinc.
10.3.4.2 External Risk Factors for Pressure Ulcer Development
Friction and Shear forces : Prolonged upright positioning and repositioning of the body without surface barriers such as a sheet can subject the body to both persistent and dynamic shear forces. Removing bed linens or diapers with the body pressed on the surface can cause significant friction and shear force injury. In terms of mitigating risk, the ability of caregivers and devices to relieve pressure adequately in immobile patients is highly associated with pressure ulcer formation and resolution. Pressure-relieving devices including foam or pillow offloaders and static and dynamic bed surfaces coupled with proper positioning and repositioning schedules can reduce risk.
Moisture : Persistent moisture from urinary incontinence, fecal incontinence, sweat or heavily exuding wounds can cause maceration and dermatitis that can increase the likelihood of dynamic friction and shear forces to induce tissue injury. Wet skin is more prone to pressure ulceration than dry skin. Persistent moisture creates weakness in dermal bonds and localized edema; alkaline urine and fecal irritants, in particular, can weaken the stratum corneum making the skin far more prone to maceration and damage in the setting of friction and pressure. Fecal incontinence of soft stool or diarrhea is more potent than urinary incontinence in causing the perineal skin to inflame and, subsequently, to break down [14, 30]. Careful attention to hygiene is therefore important to emphasize with caregivers.
10.4 Direct Care of Pressure Wounds in the Home
The key to successful wound care is a comprehensive and collaborative approach; multiple interventions need to be implemented in order to maximize success and likelihood of wound closure and the prevention of wound recurrence. Collaboration with interdisciplinary personnel, consultants, and caregivers is essential to optimal wound healing. Adequate pain control with a low threshold for opiates when acetaminophen or nonsteroidal anti-inflammatory drugs are unsuccessful is also essential. Without adequate pain control, direct wound care is not simply difficult to perform but is counter to palliative care goals so intrinsic to good home-based medical care.
The comprehensive care of wounds can be separated into several direct and indirect tasks. Direct wound care is the most relevant to the home-based medical provider and perhaps the most daunting because of poor training and misconception of such care as germane only to surgeons or wound care nurses.
The following summary of wound care is most relevant to the care of decubitus wounds. Various principles, however, can be extrapolated to the care of venous stasis ulcers, arterial ulcers, neuropathic wounds, and diabetic foot ulcers. The care of each of these wounds is more nuanced than represented below and so the discussion of such wounds follows in more detailed format.
10.4.1 Pressure Wound Assessment
Accurate classification and measurements are important for effective communication with interdisciplinary providers, monitoring for progress, and identifying appropriate therapy. The therapy can be complex and involve an array of topical treatments, debridement modalities, support surfaces, and mechanical closure devices. Classifying wounds not simply by stage but also by percentage slough, necrosis, characteristics of the surrounding tissue (“periwound”), and degree of tunneling and undermining is a critical skill for home-based providers.
Beyond staging, the following assessments should be performed for each wound:
1.
Size of the wound in length, width, and depth. It is best to use paper tape to measure. The asymmetry of many wounds means that the length and width are often not simple. We, nonetheless, recommend the following approach:
Use the clockface as the reference for communication and documentation. Use the head and feet as reference points for 12 o’clock and 6 o’clock respectively.
Measure caudal–cranial width across the longest part of the wound.
Measure lateral width across the longest part of the wound.
Measure depth of the wound with a cotton swab or the finger gently placed to the base of the wound bed. Note the degree to which deeper tissues such as tendon and bone can be visualized.
Use the cotton swab to measure the depth of undermining and note the extent along the clock-face. In addition, use the cotton swab to measure the depth of tunneling. Be as descriptive as possible; for example, “Undermining from 2 o’clock to 6 o’clock with maximum depth of 3 cm at 4 o’clock and minimum of 0.5 cm at 2 and 6 o’clock.”
2.
Determine the percentage of slough and necrosis. Necrosis, or eschar, should be noted as wet or dry and adherent or friable.
3.
Note the degree of exudate as mild, moderate, or heavy and the quality as serous, purulent, or bloody.
4.
Document the quality and extent of the peripheral wound bed or periwound. The degree and extent of maceration, visible as bleached and edematous tissue, should be noted. In addition, any erythema or hyperpigmentation (especially in dark-skinned persons), bruising, tenderness, or crepitus should be noted. Finally, scar tissue, which is vulnerable to additional breakdown, should also be recorded.
5.
Wounds should be classified as the most advanced stage by depth, and should not be reclassified as they heal. For example, as a Stage III pressure ulcer becomes more shallow, it should be described as a “healing Stage III ulcer,” not reclassified as a Stage I or II ulcer.
10.4.1.1 Wound Bed Preparation: Cleansing, Initial and Maintenance Debridement, and Primary and Secondary Dressings
The general principles of direct wound care are summarized as follows:
1.
Keep wounds with craters moist.
2.
Keep wounds with intact skin covered with either a dressing or an absorptive emollient to prevent opening. Only intact skin with clear underlying pus or crepitus should be intentionally opened.
3.
Fill dead space with packing materials tailored to the degree of exudate. Although evidence is limited, we often choose packing materials with antimicrobial activity in areas with a high-risk of infection [31].
4.
Occlude all wounds with padding necessary to absorb excess exudate and adhesives that restrict entry of urine, stool, or sweat.
5.
Keep the periwound dry with the use of barrier creams and/or skin preparations before applying adhesives.
6.
Remove senescent or dead tissue with chemical, autolytic, or sharp debridement.
Wound Cleansing : Preservative-free normal saline should be used for most chronic wounds. In general, povidone–iodine solution, hydrogen peroxide, isopropyl alcohol and sodium hypochlorite (bleach that is commercially available as Dakin’s Solution™) should be avoided given their high destruction of viable tissue and consequential delay in wound healing except in select circumstances. Povidone–iodine solution is used primarily to keep dry gangrene desiccated especially in heel ulcers whether pressure-related, neuropathic, or arterial. All arterial ulcers that are dry, in particular, should be kept dry with daily topical application of povidone–iodine solution by cotton swab or gauze [32]. Povidone–iodine use with wet wounds is limited to situations in which preparation of a sterile wound bed is necessary for sharp debridement. After the wound is debrided, the povidone–iodine solution is removed with saline. Dakin’s Solution™ is advised as a time-limited application of ¼ strength solution to foul smelling wounds just long enough to control the smell. It is very caustic and painful, so in general it is to be avoided long-term and if pain should be significant, discontinued entirely. In palliative care settings, Dakin’s Solution™ is rarely advised. On occasion, with extensive areas of dense senescent tissue (slough) or biofilm we will apply Dakin’s full strength to the wound area once to remove the tissue or film. This is particularly useful for densely adherent slough with substantial surface involvement. Prior to application, adequate analgesia is usually necessary with subcutaneous or subsurface lidocaine 1–2 %.
Initial and Maintenance Debridement: Debridement , or the removal of visible and invisible nonviable tissue, is essential to reducing infection and promoting and speeding closure. Known as wound bed preparation (WBP), debridement is a two-phase process that may or may not follow sequentially. Initial debridement consists of the primary removal of visible nonviable tissue such as fibrin slough and necrotic eschar [33]. Maintenance debridement involves the ongoing periodic removal of cellular components such as nondividing or senescent fibroblasts and keratinocytes and proteins of the extracellular matrix that can impede an advancing tissue edge and the cellular turnover necessary for tissue healing [34]. Debridement should be a continual process until complete wound closure.
Mechanical debridement is perhaps the most nonspecific type of debridement with utility in limited settings. As the process can destroy both viable and nonviable tissues, it is most useful in wounds in which the slough or eschar is extensive or in areas of extensive bacterial biofilm [9]. We describe three methods of mechanical debridement below:
Wet-to-Dry dressing debridement : As a practice, we do not employ this type of debridement in home-based settings. Saline-saturated gauze dressings are placed in a wound bed, long enough to allow the gauze to dry. The gauze is then pulled out, creating a type of mechanical debridement that is highly nonspecific for both viable and nonviable tissues [35]. As a wound care strategy, it is labor intensive, can promote a wound’s bacterial burden, is painful, and is superseded by multiple other methods of effective debridement.
Gauze debridement : Highly destructive and often painful, gauze debridement is performed with gauze moistened with saline to rub areas of slough, bacterial biofilm, or necrosis with force. It has limited use in home-based medical care except when slough, bacterial biofilm, or necrosis is semi-adherent and limited in area.
High-pressure saline or water debridement : Saline or sterile water administered to a wound under high pressure removes bacterial biofilm and cellular debris; it has more limited usefulness with semi-adherent slough and eschar. For most deep ulcers, we perform high-pressure saline irrigation to reduce poorly visible biofilm and extracellular matrix that contain senescent fibroblasts and keratinocytes, as well as collagen which can ultimately form slough. In home-based wound care, we often create an impromptu yet highly effective device by attaching a 30–60 cc syringe to a 16, 18, or 20 gauge peripheral venous cannula (with the needle removed) or the tubing of a winged-infusion set (butterfly) and clipping the tubing to approximately 5–10 cm.
Autolytic debridement involves the use of dressings and formulations that promote the body’s natural enzymes to continually remove cellular debris from the wound. In essence, all moist, occlusive dressings promote autolytic debridement, but as an exclusive means to care for the wound, autolytic debridement has limited use in treating shallow wounds.
Chemical debridement involves the application of agents that degrade or digest extracellular proteins sustaining necrotic or nonviable tissue in wound beds. This type of debridement is usually selective for senescent or dead tissue although penetration and effectiveness can be variable depending on the depth, density and moisture of the devitalized tissue. The application of nonspecific agents such as Dakin’s Solution™, is less selective for devitalized tissue and though limited in effectiveness can be useful in certain wound care settings as described below.
Enzymatic debridement : Because collagen in healthy tissue is shielded by mucopolysaccharides, only exposed collagen in devitalized tissue is susceptible to degradation by metalloproteinases contained in such preparations as papain-urea and collagenase [36]. Papain-urea combinations were removed from the US market in 2009 because of allergic reactions associated with the papaya-derived papain component. Thus, home-based medical providers are left with only collagenase for the selective chemical debridement of wounds. For maximal success in using collagenase, dense slough, or eschar should be cross-hatched with a #10 blade to achieve penetration and uplifting of the collagen fibers which anchor the base of the eschar to the wound bed. In settings where sharp cross-hatching is not possible, however, application of collagenase can still promote substantial degradation of collagen [33].
Nonenzymatic debridement : Dakin’s Solution™ , or sodium hypochlorite (bleach), is a caustic chemical that can nonetheless achieve significant debridement of necrotic eschar or slough particularly if dense [37]. It is often used at ¼ strength to limit toxicity to surrounding tissue; the gauze saturated with Dakin’s Solution™ must be changed every 12 h. On occasion, with wounds requiring more rapid debridement and in which sharp debridement is difficult to perform because of the location or high density of necrotic tissue, we use full strength Dakin’s Solution™ as a one-time application. Before dabbing the area with Dakin’s saturated gauze, we anesthetize the tissue with lidocaine to minimize the severe pain that can be associated with this application. In palliative care settings, the use of Dakin’s Solution™ is generally not recommended because it induces significant pain.
Other chemical agents exist for this purpose such as honey [38] and cadexomer iodine [39]; the benefit of these preparations is that they often come in solid or semisolid forms useful for packing or filling a wound and promoting a moist wound environment. They tend to be soothing rather than caustic to the wound bed. In addition, honey and cadexomer iodine preparations have antimicrobial properties that are particularly useful in wounds with a high burden of bacteria. Their superiority to collagenase in debriding devitalized tissue is not yet supported by the existing literature.
Sharp debridement of wounds is a useful skill for all home-based medical providers. Primary care clinicians should undergo appropriate training before executing this bedside skill. Some suggestions for resources include online training modules with videos, hands-on wound care courses with a sharp debridement skills laboratory or spending several clinical sessions working side-by-side with a clinician expert, such as a vascular surgeon, podiatrist or experienced primary care physician, or nurse with ample experience in debridement techniques. Some tips for successful bedside debridement are as follows:
1.
Provide adequate analgesia locally and systemically. Before scalpel debridement, it is important to saturate the subsurface tissue with anesthetics such as lidocaine via local injection. In addition, it is advised to premedicate patients with opiates up to 30 min prior to debridement. Advising caregivers to administer analgesics prior to arrival helps to reduce provider-waiting time.
2.
Use the right scalpel with a round rather than tapered edge such as a #15 blade or a dermal curette. The latter is more useful for less dense and less adherent yellow necrosis that usually affects fascia, muscle, or tendons.
3.
Be able to physically and visibly distinguish between nonviable and vital tissue planes by lifting the edge of the necrotic tissue edge while advancing the scalpel.
Sharp debridement is indicated when chemical debridement has been unsuccessful, when more rapid tissue closure is desired or when preparing the wound bed for negative-pressure wound therapy (NPWT). Rates of wound closure can be accelerated on the order of days to weeks over chemical or autolytic debridement alone.
10.4.1.2 When Surgical Debridement and Closure are Necessary
Even in the home-based setting, it is occasionally necessary to consult a surgeon for operative debridement. In cases of extensive necrosis or infection or in the case of expansion of a wound despite aggressive home-based measures, surgical debridement and closure may be necessary, if such intervention is consistent with the patient’s overall status and goals of care. In our practice, the majority of patients request in-home care alone, so external consultation is often difficult. On occasion, surgical consultants are able to make joint home visits to advise us on necessary next steps. For foot and ankle wounds, home-based podiatrists can also be important resources for more extensive debridement.
10.4.1.3 Bacterial Colonization, Critical Colonization, and Local Wound Infection
Reducing bacterial burden in the wound bed involves three main strategies including regular cleansing and irrigation, removing devitalized tissue, packing with antimicrobial dressings, and/or creating an acidic wound environment that inhibits bacterial proliferation [40]. Contamination and colonization of ulcers are common; several factors including bacterial burden, host immunity, and degree of devitalized tissue can cause colonization to progress to critical colonization (otherwise known as local wound infection marked by foul smell, local purulence or retarded wound healing but not generally requiring antibiotic therapy), invasive tissue infection with penetration of the surrounding healthy tissue (i.e., cellulitis or abscess) and, ultimately, systemic infection. The most common organisms invading devitalized tissue are Staphylococcus aureus, Streptococcus pyogenes, and Pseudomonas aeruginosa [41]; less common species include Proteus sp. and Escherichia coli [42].
In the event of surrounding tissue penetration or systemic infection, oral, intravenous, or intramuscular antibiotic therapy may be necessary and should be tailored to the most likely organisms in the affected area. For instance, sacral wounds may require enhanced coverage of Gram-negative species. Although not routinely recommended, if a wound bed is suspected to have a high bacterial burden given persistently foul smell, slow wound healing, persistent exudate on the order of days to weeks, or frank pus, sampling for culture can guide antibacterial therapy and uncover highly resistant species.
10.5 Common Primary Dressings, Secondary Dressings, and Adhesives in Home-Based Wound Care
In general, the choice of wound care dressings should be tailored to the appearance of the wound bed, the size and depth the degree of tunneling and undermining, the degree and quality of exudate, the degree and quality of devitalized tissue, and the appearance of the periwound. When ordering and documenting dressings, it is best to characterize layers as primary (i.e., directly on top of the wound), secondary (the layer directly adjacent to the primary layer), and tertiary (anchoring or adhesive) dressings.
The following tables summarize some of the common dressings and adhesives used in home-based settings with indications for each. This table is not meant to be restrictive or exhaustive, rather it should give the clinician a general repertoire and vocabulary for various dressing types. Home-based medical care providers should note that limitations in formulary are often be imposed by the nursing agency; some nursing agencies favor certain brands over others. Because the care of decubitus wounds is often similar to other wounds, we have used pressure ulcers as the standard indication and have noted when certain dressings are also indicated for other wounds (Tables 10.1 and 10.2).
Table 10.1
Primary dressings in home-based wound care
Primary dressing | Primary forms | How it works | Optimal uses | Other indications and contraindications |
|---|---|---|---|---|
Hydrogels | Manufactured as amorphous gel or gel sheets | Lubricates and softens tissue | Shallow wounds (depth less than 1 cm) with minimal exudate | Not recommended for moderately to heavily exudative wounds or venous stasis ulcers |
Promotes autolytic debridement | Deeper wounds (depth greater than 1 cm) with significant granulation tissue and without dense necrotic tissue or slough | Can be mixed with 2 % lidocaine gel or morphine liquid for additional analgesia | ||
Wounds with thin layers of necrosis or slough in preparation for sharp debridement | Ideal for palliative care settings as frequency of dressing changes can be up to 1 week; users are advised to refer to manufacturers insert of each product for details | |||
Painful wounds in which a cooling effect is desired | ||||
Hydrocolloids | Manufactured as sheet, powders, and pastes, the latter with more absorptive capacity | Hydrophilic colloid particles bound to polyurethane which absorbs fluid from the wound and creates a gel-like covering | Well granulating wounds with mild exudate | Not recommended for use in deep wounds, infected wounds or venous stasis ulcers |
Over dry wounds acts as an impermeable barrier to moisture and a cushion against friction | Shallow wounds with thin necrosis or slough in preparation for sharp debridement | Often used as secondary dressing for Stage II or shallow Stage III ulcers | ||
Over bony prominences subject to repeated trauma from friction or shear stress such as the sacrum | Advised in palliative care settings as frequency of dressing changes can be up to a week | |||
Alginates | Manufactured as pads and ribbons. Pads can also be cut into ribbons | The absorption of wound fluid causes the seaweed-derived fibers to form a gel that promotes a moist wound environment | Given their high absorptive capacity, alginates are ideal for heavily exuding wounds | Not recommended for use in wounds with low drainage as the dressing can dry out the wound or impregnate into the wound causing difficulty in removing residual fibers |
Ideal for filling large craters, tunnels and undermining | Dressing frequency can be every other day | |||
Excellent primary dressing for venous stasis ulcers | For wounds with minor bleeding, alginates can induce hemostasis | |||
Can be used with antimicrobial gels in infected wound beds | ||||
When impregnated with silver, offers additional barrier to bacterial growth | ||||
Hydrofibers (aka hydrocolloid fiber) | Manufactured as pads and ropes; many come impregnated with silver | Like an alginate, the absorption of wound fluid causes this synthetic carboxymethylcellulose (CMC) fiberto create a gel (similar to a hydrocolloid dressing) with enhanced absorption over alginates [43] | Same as alginates | Non-recommended uses are the same as alginates |
Benefit over alginates is that the frequency of wound care dressings can be several days longer [44] | ||||
Foams and hydropolymer derivatives | Manufactured as sheets (conformable or shaped to body part), sheets with peripheral adhesives or ropes; may be impregnated with silver or other antibacterial products that inhibit colonization | Foam dressings are composed of a polymer or urethane foam with craters that trap the exudate. The polyurethane coating enhances the creation of a moist wound environment thus promoting autolytic debridement or chemical debridement when coupled with an enzymatic ointment. Hydropolymers offer enhanced absorption with a backing layer that has breathability allowing for significant evaporation of wound exudate [45]. Differing antibacterial properties of impregnated and manufactured types are useful for limiting the bacterial burden of wounds in high-risk areas, wounds with foul odor or wounds that are chronically colonized | A primary dressing for any sized cavity with moderate to heavy exudate | Polyurethane foam dressings with high moisture-vapor transmission rates (MVTR) may allow for more days between dressing changes for highly exudative wounds |
A secondary dressing for heavily exudative wounds; may be placed on top of the primary dressing; the latter can include alginates or hydrofibers | Foams are not indicated as primary dressings for dry wounds or wounds with minimal exudate as they may stick to the wound bed and removal may cause trauma or pain | |||
A secondary dressing for non-exudative wounds to limit shear force during the healing process | ||||
Primary or secondary dressing for venous stasis ulcers | ||||
Primary or secondary dressing for friable or painful neuropathic foot wounds | ||||
Gauze | Dry gauze sheets or bandages, petroleum-impregnated gauze, iodine-impregnated gauze strips, elastic gauze bandage | Gauze sheets trap moisture through the lattice; layering multiple sheets maximizes absorption | Petroleum-impregnation offers a non-stick and less painful variation that can promote a moist wound bed with varying occlusive properties
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
