(1)
Institut Bergonié, Bordeaux, France
Abstract
The term Wnt was forged by contraction of Win gless-type mouse mammary tumour virus int egration site after the discovery on a viral oncogene in mice which was homologous to a mutant Drosophila gene. The Wnt pathway, especially under its canonical form (Wnt–β-catenin pathway), is a signalling pathway involved in vertebrate and invertebrate development. It plays a major role in embryogenesis and morphogenesis and in stem cell commitment to differentiation or proliferation. This pathway is relatively complex, and all its processes and physiological roles are not completely deciphered. The proteins involved in this pathway are called ‘Wingless’, ‘Frizzled’, ‘Dishevelled’, which originally designated the mutants of Drosophila development and demonstrates the universality of this pathway in the animal kingdom. Germline alterations of this pathway in humans determine congenital hereditary diseases, and some somatic and germinal alterations are associated with oncogenesis.
Briefly, protein messengers called WNT activate GPCR-related membrane receptors called Frizzled (FZD), and this activation leads to the stabilisation of the cytoplasmic form of a protein involved in intercellular junctions of epithelial tissues, β-catenin. The stabilised protein can then be transferred into the nucleus where it activates transcription programmes of numerous genes, especially those encoding the proteins required for cell proliferation and cell cycle entry (cyclin D, MYC, etc.).
7.1 WNT Ligands and Their Receptors
Nineteen distinct WNT proteins are found in humans. The first of these genes, WNT1, had been identified at the integration site of a retrovirus inducing mammary tumours in mouse and called Int2, showing thus a potential role of this gene in normal development and oncogenesis; an ortholog of this gene, called Wingless, had been thereafter identified in Drosophila: the Wnt name results from the contraction of Wingless and Integration.
WNT proteins are cysteine-rich glycoproteins secreted by diverse cell types; their genes are organised in several clusters localised on different chromosomes and present high homology. Basic gene sequences are well conserved in all metazoans. WNT proteins are expressed in a tissue-specific manner, but the precise role of each WNT protein is yet poorly understood.
WNT ligands are recognised by Frizzled receptors (FZD); there are 10 FZD receptors in humans, but the combinatorial pattern between the 19 ligands and the 10 receptors is not entirely known. FZD receptor activation requires the presence of a phosphorylable coreceptor, LRP5 or LRP6 (LDL receptor-related protein). FZDs are seven-transmembrane domain receptors or GPCRs (G-protein-coupled receptors, Chap. 6); the concomitant activation of an FZD receptor with LRP5/6 coreceptor leads to the formation of a complex with a cytoplasmic protein called Dishevelled (DVL), an adapter protein whose activation allows the break of a cytoplasmic complex including the central element of this pathway, β-catenin. Three DVL proteins, DVL1, 2 and 3, exist in humans. It was believed that the FZD receptors function without coupling with a large heterotrimeric G-protein, contrasting to other GPCRs. It seems in fact that such G-proteins operate between FZD and DVL activation, similarly as they operate downstream the activation of all GPCR.
7.2 Wnt–β-Catenin Pathway
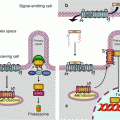
β-catenin (gene CTNNB) is a molecule involved in both cell adhesion and signalling. In the first function, β-catenin is bound, in the cytoplasm, to the actin cytoskeleton via a molecule of α-catenin and, in the extracellular space, to the junctional structures of E-cadherin (CDH1) (Fig. 7.1) (Chap. 11). β-catenin plays, therefore, a major role in adherens junctions of epithelial cells and in the maintenance of tissue architecture.
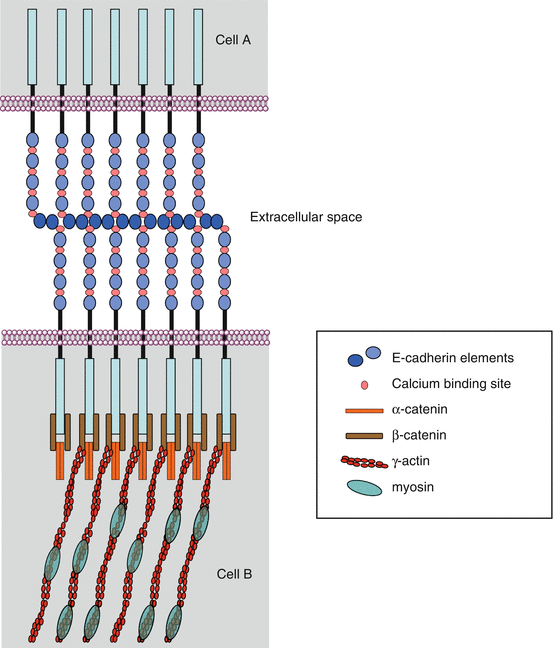

Fig. 7.1
Intercellular junctions involving E-cadherin. Two types of epithelial cell intercellular junctions involve E-cadherin: adherens junctions and desmosomes. These intercellular junctions mobilise β-catenin, which allow the anchoring of E-cadherin to the actin cytoskeleton, via a molecule of α-catenin. The E-cadherin molecules are associated in the extracellular space via Ca2+ ions; their intracellular domain is attached to β-catenin and α-catenin molecules, which tie them to the actin cytoskeleton
Free cytoplasmic β-catenin, which is in equilibrium with bound β-catenin at adherens junctions, is maintained in an inactive state, in the absence of WNT signals, thanks to its phosphorylation, which drives it to proteasomal destruction. This phosphorylation is ensured within a degradation complex, in which intervene adapter proteins, axin and APC (adenomatous polyposis coli), and two serine/threonine kinases, CK1α (casein kinase 1α, gene CSNK1A) and GSK3β (glycogen synthase kinase 3β, gene GSK3B), which can add phosphate groups on two different sites. β-catenin phosphorylation enables its binding to β-TRCP (beta-transducing repeat-containing protein), which is its cognate E3 ubiquitin ligase and drives it to proteasomal destruction (see Annex C) (Fig. 7.2a).
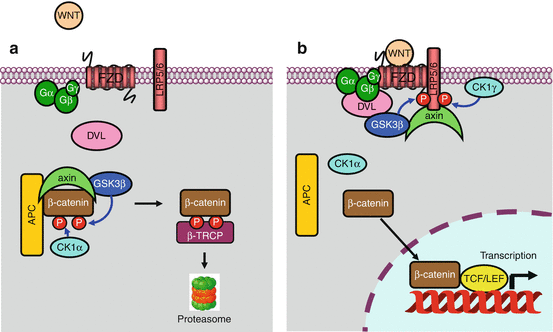

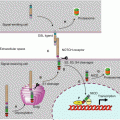
Fig. 7.2
The Wnt–β-catenin pathway. (a) In the absence of stimulation of the Frizzled (FZD) receptor by a WNT ligand, β-catenin is phosphorylated in a destruction complex-containing axin, APC and the GSK3β and CK1α kinases. This phosphorylation leads β-catenin to its E3 ubiquitin ligase, β-TRCP, and then to the proteasome. (b) In the presence of a WNT ligand bound to the FZD receptor, a dishevelled (DVL) protein is recruited, likely after activation of a large heterotrimeric G-protein, allowing thus the phosphorylation of the LRP5/6 coreceptor by CK1γ and GSK3β and the recruitment of axin to the membrane. The destruction complex is dissociated, and non-phosphorylated β-catenin can enter the nucleus, where it associates to a transcription complex including the TCF/LEF factors. The derepression of this complex allows the transcription of numerous genes involved in cell proliferation, among which MYC and CCND1 are relevant examples
Frizzled receptor-induced DVL activation, likely through a large heterotrimeric G-protein, induces the phosphorylation of the coreceptor LRP5/6, on a PPPSPXS motif, by a membrane casein kinase (CK1γ, gene CSNK1G1) and by GSK3β, which can no longer phosphorylate β-catenin (Fig. 7.2b). This phosphorylation generates a signal that induces the relocalisation of axin to the membrane, releasing then β-catenin from the cytoplasmic destruction complex which was until then driving it to the proteasome in the absence of receptor activation. This Wnt pathway, called the ‘canonical pathway’, appears to be most often activated after receptor binding of specific WNT ligands, WNT1, WINT3A and WNT8.
Free, non-phosphorylated β-catenin can then enter the nucleus, where it is able to move a transcription factor of the TCF/LEF (T-cell factor/lymphoid enhancer factor) family from its DNA binding sites and thus trigger the transcription of genes that were until then repressed (Fig. 7.2b). Among the genes that are thus transcribed are MYC, which encodes a transcription factor involved in cell proliferation (Chap. 2), and cyclin D1 (CCND1), which is involved in cell cycle entry (Chap. 17). When it gets out of the nucleus, β-catenin is recaptured by APC and, in the absence of a new WNT signal, reinserted into a destruction complex.
Other systems appear to be able to indirectly activate β-catenin pathway, by inducing the break of the β-catenin–E-cadherin bond and the increase in cytoplasmic β-catenin availability for nucleus entry; this is achieved by tyrosine phosphorylation of β-catenin by tyrosine kinase receptors such as EGFR, ERBB2 or MET (Chap. 1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree