Fig. 20.1
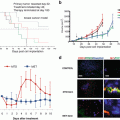
Drug efficacy testing in the PDOX model of HER-2-expressing cervical carcinoma. PDOX nude mice were treated with saline, carboplatin, trastuzumab + lapatinib, trastuzumab + lapatinib + entinostat, or entinostat alone. (a) Bar graphs of the primary tumor volume in each group. (b) Bar graphs of the metastatic tumor weight in each group (n = 5 for each treatment arm). *p<0.05; **p<0.01 [4]
These results are another important example of how the tumor site can have a major effect on drug sensitivity. In particular, primary and metastatic tumors may have dramatically opposite patterns of drug sensitivity. Orthotopic, rather than sub-cutaneous mouse models of cancer, since they can metastasize, are necessary to determine accurate drug response, especially when used for designing patient treatment.
Materials and Methods
Tumor Implantation
For the cervical cancer model, subcutaneous tumors grown in nude mice, were harvested and divided into small fragments for orthotopic transplantation. A small 6–10 mm midline incision was made on the lower abdomen of the nude mouse through the skin and peritoneum. The uterus was exposed through this incision, and a single 3 mm3 tumor fragment was sutured to the cervix of the uterus using 8-0 nylon surgical sutures (Ethilon; Ethicon Inc., NJ, USA). On completion of tumor implantation, the uterus was returned to the abdomen, and the incision was closed in one layer using 6-0 nylon surgical sutures (Ethilon) [18] (see Chap. 10 in the present volume).
For the CT26 model, Wilmanns et al. [6] produced s.c. tumors by injecting (2.5 × 104) CT- 26 cancer cells in 0.05 ml HBSS with a 27-G needle into the lateral flank. Wilmanns et al. [6] produced spleen tumors and experimental liver metastasis in anesthetized mice by making a small incision in the body wall to examine the spleen. CT-26 cancer cells (2 × 104) were injected with a 27-G needle into the spleen parenchyma. Wilmanns et al. [6] produced cecal wall tumors after methoxyflurane anesthesia by making a small incision to exteriorize the cecum and injecting CT-26 cells between the submucosa and the subserosa. Wilmanns et al. [6] produced experimental lung metastasis by injecting in the tail vein 2 × 104 CT-26 cells in 0.2 ml HBSS [6].
Furukawa et al. [10] orthotopically transplanted gastric tumor-tissue pieces of approximately 3 × 3 × 3 mm in nude mice. Mice were anesthetized with a 2.5% solution of a mixture of 2,2,2-tribromoethanol (Sigma-Aldrich) and tert-amyl alcohol (1:1). An incision through the left upper abdominal pararectal line and peritoneum was made. The stomach was exposed and a part of the serosal membrane on the middle of the greater curvature of the glandular stomach (2 mm) was mechanically injured with scissors. Tumor pieces were fixed on injured sites of the serosal surface with 6-0 Dexon transmural sutures [10].
Treatment
Treatment Protocol for the Cervical Cancer Subcutaneous and PDOX Models
Six weeks after implantation, the mice in the PDX and PDOX models were randomized and treated in the following groups of n = 5: (1) saline control, (ip, weekly, 5 weeks); (2) carboplatin (Selleck Chemicals, Houston, TX, USA, 30 mg/kg, ip, weekly, 5 weeks); (3) trastuzumab (Genentech, Inc., South San Francisco, CA, USA, 20 mg/kg, ip, weekly, 5 weeks) + lapatinib (Selleck Chemicals, 100 mg/kg, orally, daily, 5 weeks); (4) trastuzumab (20 mg/kg, ip, weekly, 5 weeks) + lapatinib (100 mg/kg, orally, daily, 5 weeks) + entinostat (Selleck Chemicals, 5 mg/kg, orally, daily, 5 weeks); and (5) entinostat (5 mg/kg, orally, daily, 5 weeks) [4].
Treatment Protocol for the Orthotopic Colon Cancer Model
Wilmanns et al. [6] administered doxorubicin (DOX) in the lateral tail vein on days 7 and 16 after tumor cell injection at a dose of 10 mg/kg body weight. Wilmanns et al. [6] dissolved 5-FU in PBS to a concentration of 2 mg/ml which was injected at a dose of 20 mg/kg body weight in the lateral tail vein. Wilmanns et al. [6] administered 5-FU in five daily injections followed by weekly injection.
Treatment Protocol for the Orthotopic Gastric-Cancer Model
Furukawa et al. [10] dissolved 5-FU and MCC in a physiological saline solution and administered the drugs i.p. as a bolus. 5-FU was administered at 180, 90, and 45 mg/kg and MMC was administered at 6, 3, and 1.5 mg/kg which were equivalent to maximum tolerated doses (MTDs), half MTDs, and quarter MTDs in nude mice. Furukawa et al. [10] administered OK-432 i.p. everyday for 5 days from day 5 to day 9 at a dose of 1 Klinische Einheit (KE) per mouse.
Conclusions
The early Fidler et al. [6] and Furukawa et al. [10] papers showed that primary and metastatic tumors can have differential chemosensitivity. In the cervical cancer PDOX model, entinostat monotherapy significantly inhibited the metastasis of HER-2-positive cervical cancer even though there was no efficacy of this agent on the primary tumor or on the subcutaneous model. The efficacy of entinostat would not have been detected in a subcutaneous PDX model of this tumor [4]. These results emphasize that patient mouse models of cancer should be orthotopic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree