Histology
Initial stage
Therapy
Relapse rate (%)
Relapse rate >2 years
Main area of relapse
Reference
Seminoma
I
Surveillance
12–31
3–5 %
Abdomen
1 % >3 years
Seminoma
I
Adj. carboplatin
5
1 %
Abdomen
Seminoma
I
Adj. RT 20 Gy
4
1 %
Outside RT field
[21]
Seminoma
IIA/B
RT 30/36Gy
5–15
2 %
Outside RT field
[22]
Seminoma
IIC–III, good prognosis
3xBEP/4xEP
8–12
<2 %
Abdomen, lung
Nonseminoma
I, low risk
Surveillance
15
1 %
Abdomen, lung
Nonseminoma
I, high risk
Surveillance
45–50
<2 %
Abdomen, lung
[17]
Nonseminoma
I
Adj. RPLND
8–10
<2 %
Lung
[26]
Nonseminoma
I, high risk
Adj. 1xBEP
3–4
<1 %
Abdomen, lung
[27]
Nonseminoma
IIA–III, good prognosis
3xBEP/4xEP
8–12
<2 %
Abdomen, lung
It is important to note that patients diagnosed with either intermediate- or poor-prognosis disease according to IGCCCG [28] or patients who did never reach a complete remission are not eligible for standard follow-up as will be outlined in the following paragraphs. These patients should receive individualized care under the lead of an experienced high-volume center.
This chapter reviews the recent recommendations for the follow-up of patients with testicular cancer and discusses the risk associated with ionizing radiation.
10.2 Modalities of Follow-Up
10.2.1 General Recommendations
The follow-up of testis cancer patients should be performed by a physician who has profound knowledge of this type of rare cancer and experience in its treatment.
A complete medical history and examination is the cornerstone of each follow-up visit. Changes in weight, increased fatigue, new onset of pain (especially in the abdomen or back), cough, dyspnea, as well as erectile function and libido should be assessed specifically [10, 29, 30]. The clinical exam includes measurement of height and weight (or waist circumference), blood pressure, auscultation of the lungs, palpation of supra- and infradiaphragmal regional lymph node regions (cervical, supraclavicular, axillary, inguinal), and palpation of the remaining testis [2, 29, 30].
Measurement of the serum tumor markers AFP (alpha-fetoprotein), β-hCG (β-human chorionic gonadotropin), and LDH (lactate dehydrogenase) is central in the follow-up of testis cancer patients [2, 29, 30]. If possible, the tumor markers should always be checked in the same qualified laboratory. AFP is only elevated in patients with nonseminomatous germ cell tumors, whereas β-hCG can also be increased in up to 20 % of patients with seminoma [30]. The initial presentation does not predict whether the patient will have elevated markers at relapse: initially, marker-positive tumors can have a marker-negative relapse and vice versa [30]. Therefore, to control all tumor markers is the standard of care also in patients with marker-negative tumors at diagnosis. The role of LDH in the follow-up schedule is debatable. It has limited sensitivity and specificity, and a high rate of false-positive tests is found. However, some publications show that it can contribute to identify recurrence especially in advanced cases [31]. Cautious interpretation of LDH is however necessary.
10.2.2 Choice and Extent of Imaging Modality
There is general consensus that all patients have to be staged with a CT scan of the thorax, abdomen, and pelvis at the initial diagnosis of testis cancer [7–9, 30]. In case of poor-prognosis metastatic disease or presence of neurological symptoms, a magnetic resonance imaging (MRI) of the brain should be performed [30]. A bone scintigraphy is reasonable in case of specific bone pain, but bone metastases are very rare.
In contrast to the consensus at initial diagnosis, the choice, the extent, and the frequency of imaging in the follow-up setting are less clearly defined. For many years, repeated CT scanning with up to >20 CT scans during follow-up of a testis cancer patient was standard practice. Over the recent years, the risks of ionizing radiation associated with CT scans have been recognized and have changed the approach and attitude toward the use of CT scans in the follow-up setting. The risks of ionizing radiation will be discussed in Sect. 10.2.3.
Only very few publications have focused on the modalities of imaging in the follow-up of testis cancer. Retrospective studies looked at the usefulness of regular CT scans of the pelvis or the chest [32, 33], and some studies evaluated the necessity of regular chest x-rays [34, 35]. With regard to CT of the pelvis, only a small group of patients is at increased risk of recurrence in the pelvis only: this includes patients with bulky abdominal disease (>5 cm), previous history of maldescent of the testis or orchidopexy, history of previous scrotal surgery, and invasion of the carcinoma into the tunica vaginalis of the testis [32]. Moreover, patients who have been treated for seminoma stage I with para-aortic radiotherapy can present with isolated pelvic recurrence. Only in these particular cases should a CT of the pelvis be included in the follow-up schedule [32]. Regarding the CT of the thorax, a retrospective analysis in patients with stage I nonseminoma revealed that all recurrences were diagnosed with elevated tumor markers, abdominal disease, or lesions visible on a conventional chest x-ray [33]. It is generally accepted that regular CT of the thorax is not necessary in the follow-up of testis cancer patients. The necessity of regular chest x-rays has also been debated in some publications [34, 35]. In contrast to a chest CT, the conventional chest x-ray (especially if performed only as posteroanterior image) applies a very low amount of ionizing radiation (0.02 mSv), is easily performed, and is cheap. Based on these considerations, the use of regular chest x-rays has been reduced in recent recommendations but not abandoned. There is clear consensus that positron emission tomography (PET) CT is not indicated in the follow-up of testis cancer patients and should not be used.
Instead of using CT for abdominal imaging, magnetic resonance imaging (MRI) of the abdomen can be a suitable replacement. Some experts, namely, the SWENOTECA group, have replaced abdominal CT by abdominal MRI [36]. MRI does not carry any risk of ionizing radiation and can be performed in patients with allergic reactions to iodine contrast agents used with CT scans. Limited resources and the lack of experience in interpreting the MRI scan currently restrict its use. In experienced centers with enough resources and expertise, the abdominal MRI can replace the CT.
The German Testicular Cancer Study Group (GTCSG) included the use of abdominal ultrasound in addition to the recommended scanning with CT in their recommendations [2, 3]. Abdominal ultrasound is highly dependent on the experience of the examiner and the anatomy and preparation of the patient. Ultrasound is therefore not recommended in case of obese patients or if performed by not appropriately trained physicians. In a small study, ultrasound appeared to have similar sensitivity and specificity for the detection of retroperitoneal metastases in patients with testicular cancer when compared to a CT of the abdomen [37]. The ultrasound should not replace all CT scans planned in the schedule but can be helpful in the long-term follow-up after 5 years to detect slow-growing teratoma and in cases where the patient requests a more intense follow-up schedule.
10.2.3 Ultrasound of the Remaining Testis
The use of regular ultrasound of the remaining testis is a subject of controversial discussion. Patients under the age of 40 years with a low testis volume (<12 ml) are at higher risk of developing a contralateral tumor. Biopsy should be offered in these cases [30]. In case of contralateral testis biopsy without evidence of carcinoma in situ (CIS, also known as intratubular germ cell neoplasia) or after radiotherapy for CIS, the risk of a contralateral tumor is much lower but not eliminated. Some guidelines recommend an annual ultrasound of the testis for 10 years, especially if no biopsy was performed [2]. Regular self-examination by the patient should be highly encouraged, and the remaining testis should be palpated by the physician at every visit. An ultrasound should be performed in case of any suspicious findings.
10.2.4 Follow-Up for Long-Term Toxicity
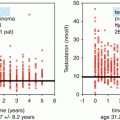
Patients who have been cured with chemotherapy or radiotherapy may develop late toxicity. This includes cardiovascular disease, metabolic syndrome (arterial hypertension, impaired glucose tolerance, hyperlipidemia, obesity), impaired renal function, ototoxicity, neuropathy, Reynaud’s phenomenon, as well as hypogonadism and secondary malignancies [10–14]. Regular checkup of blood pressure, weight, body mass index, or waist circumference as well as blood lipids (total cholesterol, LDL-cholesterol, triglycerides) is recommended at baseline and annually afterward. Patients should be followed for hormonal imbalances (total testosterone, LH, FSH) 1 year after diagnosis and then regularly every 1–2 years. In case of pathological findings or a suggestive history of hypogonadism (e.g., missing morning erection), the hormonal status should be determined repeatedly on an individual basis. In case of symptomatic testosterone deficiency, substitution has to be discussed.
Testis cancer survivors should regularly be advised to adapt to a healthier lifestyle in order to control additional risk factors (e.g., nonsmoking, weight control, regular physical exercise).
An extensive review of the current literature on long-term toxicities and survivorship issues can be found in Chap. 11.
10.3 Risks Associated with Ionizing Radiation
In the recent decades, a rapid increase in the use of medical imaging with ionizing radiation has been noted. Over the last 30 years, the annual per capita effective dose has increased about sixfold (from 0.5 mSv in 1980 to 3.0 mSv in 2006) [38]. The radiation dose from medical sources now exceeds the natural background radiation (background radiation approximately 2.4 mSv). Computed tomography and nuclear imaging are responsible for over 75 % of ionizing radiation administered [39]. Of these, CT scans of the abdomen, the pelvis, and the chest account for 18.3, 12.2, and 7.5 % of the total effective dose from all medicinal imaging procedures, respectively. The effective dose administered per exam differs from one procedure to another [39, 40]. Typically, the average effective dose from an abdominal CT is around 8 mSv per exam which is comparable to the natural background radiation for 3 years [40]. Table 10.2 shows the average effective doses for different CT scans. In comparison, a chest x-ray (posteroanterior) applies only 0.02 mSv of ionizing radiation [39]. It is important to note that there is inhomogeneous distribution in different organs and that, hence, the amount of radiation and risk for certain organs can differ [6, 41]. With the evolution of more modern CT scanners, the average effective dose per examination has increased over the last decades [42]. On the other hand, radiation reduction strategies have been tested in recent years including the use of lower radiation dose, lower voltage, and smaller volumes. Some of these improvements have however not been implemented in all radiology departments and others remain experimental. Therefore, the mean effective dose for each type of CT can vary considerably (up to 13-fold higher doses) at different sites [43].
Table 10.2
Effective doses from various CT scans in mSv
Examination | Average effective dose (mSv) | Values reported in literature (mSv) |
|---|---|---|
Head | 2 | 0.9–4.0 |
Chest | 7 | 4.0–18.0 |
Abdomen | 8 | 3.5–25.0 |
Pelvis | 6 | 3.3–10.0 |
The excessive risks of radiation exposure due to a single or repeated computed tomography (CT) have been calculated [6, 41]. These calculations are based on stochastic risk calculations representing the statistical risk of genetic damage occurring with radiation exposure. The stochastic risk is calculated from information based on cohort studies from atomic-bomb survivors [44]. The radiation exposure of each CT carries a small carcinogenic risk which is higher the younger the patient is. Moreover, repeated CTs lead to an increased cumulative risk [6]. Calculations have been published showing that a single CT of the abdomen performed in a 20-year-old male can lead to a radiation-induced cancer in 1 of 660 cases [43]. For one chest CT, this number is 1 in 1,020 men [43]. While these calculations are discussed controversially, the FDA clearly states that there is a lifetime risk of cancer attributed to ionizing radiation with a particular risk for younger patients [45].
A research group at Stanford University estimated the risk of developing cancer due to ionizing radiation from imaging for patients with stage I testis cancer undergoing surveillance according to the NCCN protocol with up to 16 scans: the lifetime cancer risk induced by CT scans for follow-up purposes ranged from 1.2 % in a 40-year- old to 1.9 % in an 18-year-old patient and increased to 2.6 % if chest CT was included [46]. These numbers outweigh the benefit of surveillance where the overall survival from testis cancer is nearly 100 %. In contrast, a Canadian group looked at the association of secondary malignancies in patients with testis cancer who had a median of 10 abdominopelvic CT scans during their follow-up [47]. They could not find any increased risk for second abdominopelvic malignancies. However, this study has been heavily criticized due to the fact that the medium follow-up was only 11 years, while it is clearly recognized that radiation-induced malignancies only develop 30–40 years after exposure [13, 14].
Minimizing the amount of ionizing radiation remains an important goal in the management of patients with testis cancer who are often young and therefore more vulnerable. Methods to reduce the radiation burden include technical improvements, reducing the scanned volume, reducing the frequency of scanning, and moreover a switch from CT scans to other imaging modalities such as MRI or ultrasound.
10.4 Active Surveillance for Stage I Testis Cancer
Most patients with testicular cancer are diagnosed with stage I disease localized in the testis: 80 % of seminoma and 60 % of nonseminoma patients present with stage I [48]. The optimal management of stage I disease is undergoing constant change: In recent years, there has been a shift toward increased use of active surveillance in stage I disease for seminoma and nonseminoma [49]. Several reports have shown that active surveillance is safe for patients and associated with excellent outcomes [16, 17, 25, 36]. Data from the United States report that active surveillance is now the preferred mode of treatment for stage I testis cancer [50]. The basis for successful active surveillance is early detection of recurrence while minimizing ionizing radiation and overall treatment burden. The optimal adherence of the patient and the physician to a proposed schedule is essential for the success of active surveillance.
Because seminomatous and nonseminomatous stage I germ cell tumors differ considerably with regard to time and site of recurrence, these two entities will be discussed separately in the following.
10.4.1 Active Surveillance for Patients with Seminomatous Germ Cell Tumor
For stage I seminomas, the recurrence risk after orchiectomy is around 15–20 %. Adjuvant treatment with para-aortic radiotherapy or chemotherapy with single-dose carboplatin reduces the risk of recurrence to 4–5 % [19–21]. Active surveillance and adjuvant treatment are equal treatment options regarding overall outcome according to published guidelines [7–9]. The advantage of active surveillance lies in the fact that 80–85 % of patients can avoid unnecessary treatment. There is an ongoing debate as to whether seminoma stage I patients can be grouped into different risk categories: a retrospective analysis suggested that patients with tumors >4 cm in size and with invasion of the rete testis had a significantly increased relapse rate of up to 32 % [18]. However, a prospective analysis from the same group as well as results from the SWENOTECA group could not confirm these risk categories [36, 51]. Large prospective cohorts of seminoma stage I patients treated with active surveillance have been reported [16, 36]: they show that 75 % of patients relapse within 2 years, but a further 15–20 % relapse in year 3. With regard to the whole population, < 1 % of patients relapse after 3 years [16]. The large majority of relapses occur in the abdomen and are detected on abdominal imaging; a minority of patients are primarily diagnosed with elevated tumor markers. Due to this pattern of recurrence, some authors have suggested that regular measurement of tumor markers or chest x-rays is unnecessary in active surveillance of seminoma [34, 52]. All recommendations however continue to advise regular use of tumor markers and x-rays, also in an attempt to maintain patient adherence. The major controversy in stage I seminoma concerns the interval and modality of abdominal imaging. A trial addressing this very important question is being performed in the United Kingdom and will soon complete accrual (TRISST) [53]: in this 4-arm trial, 3 vs. 7 scans and CT vs. MRI are evaluated. Currently, most groups advise 4–6 CT scans within the first 3 years. Further regular imaging after 3 years is debated due to the very low risk of recurrence. Table 10.3 lists the recommendations of three different organizations for patients with seminoma stage I undergoing active surveillance.
Table 10.3




Comparison of different recommendations for active surveillance in seminomatous germ cell tumor stage I
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree