Fig. 11.1
Cold-induced Raynaud phenomenon of the fingers in a cisplatin-treated TCSs
11.6 Neurotoxicity
Determination of neurotoxicity is a complex task and most precisely performed by neurophysiologic examinations, which are time and cost consuming. A short 6-item questionnaire, called SCIN (scale for chemotherapy-induced long-term neurotoxicity), covers paresthesias and Raynaud’s phenomena in the hands and feet, as well as ototoxicity, i.e., tinnitus and hearing impairment, and might help to quantify these complications [37]. Using this scale, paresthesias were stated by 29 % of cisplatin-treated TCSs as opposed to 10 % after surgery only [38]. Application of five or more cycles increases the proportion of those bothered by paresthesias to 46 %. The observed large interindividual variations of neurotoxicity may partly rely on polymorphisms of the detoxifying enzyme glutathione S-transferase P1 (GST-P1) [39]. Platinum is measurable in the serum of TCSs many years after its application, and the intensity of paresthesias is more strongly associated with these serum levels than with the cumulative dose of cisplatin [40]. Intriguingly, the association between serum platinum levels measured 10 years after application was strongly associated with subsequent self-reported paresthesias 20 years after treatment than with concomitantly reported paresthesias, indicating an ongoing neuronal damage. Unfortunately, the growing insights into the pathophysiology have not yielded preventive or palliative treatments. Particularly vulnerable TCSs, who do not benefit sufficiently from anticonvulsant drugs like pregabalin, may require morphine for pain relief. A Cochrane Database review concluded that the use of neuroprotective agents, such as acetylcysteine, amifostine, calcium and magnesium, diethyldithiocarbamate, glutathione, Org 2766, oxcarbazepine, or vitamin E, could not be recommended since no substance had prevented or limited neurotoxicity of platinum drugs [41].
11.7 Ototoxicity
Cisplatin-induced ototoxicity is a distinct side effect of cisplatin and presumably caused by selective damage to the outer hair cells [42]. Cisplatin-induced ototoxicity, i.e., tinnitus and hearing impairment, shows large interindividual variations [43]. Hearing ability of high frequencies of 4,000 Hz and above is typically affected, and these changes persist for many years [44]. Some TCS are not very bothered by the typical loss of high-frequency hearing which is similar to the aging-related presbycusis, since language communication is based on lower frequencies. Hearing impairment and tinnitus are probably rather caused by cisplatin’s peak concentration than of its cumulative dose since application of 50 mg/m2 cisplatin over 2 days as compared to 20 mg/m2 over 5 days resulted in ORs of 5.1 and 7.3, respectively [38]. Unfortunately, there is no remedy for the prevention of cisplatin-induced ototoxicity, leaving no room for more specific recommendations than to avoid noise and loud music.
11.8 Nephrotoxicity
Both radiotherapy and cisplatin-based chemotherapy lead to a reduced renal function in 20–30 % of TCSs [44–47], and a decreased GFR is measured in up to 30 % of asymptomatic TCSs [46]. Hypomagnesemia may be observed after cisplatin application [48], but its long-term persistence and potential clinical implications are not unequivocally established [49]. Impaired renal function is considered an important complication since even moderate deteriorations have an adverse impact on CVD and all-cause mortality in the general population [50]. In testicular cancer patients, reduced renal elimination of cisplatin and bleomycin might increase the risk of other toxicities, e.g., bleomycin-related pneumonitis [51, 52].
11.9 Hypogonadism
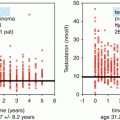
Testicular endocrine dysfunction comprises insufficient testosterone (T) production and/or compensatory increased luteinizing hormone (LH) levels. Subnormal testosterone levels have been reported in TCS treated with chemotherapy compared with surgery only or the general population [29, 53]. Furthermore, low testosterone levels are associated with increased risks for the metabolic syndrome and CVD [29, 33, 34, 37], as well as decreased pulmonary function in TCS [20]. Of note, low testosterone levels are also associated with increased prevalence of the metabolic syndrome and an increased risk for CVD mortality [54, 55], reduction of spirometry variables [56], and increased risk of respiratory disease mortality [57] in epidemiologic studies. Testosterone treatment has been shown to improve lipid profiles and insulin resistance in men with diabetes or the metabolic syndrome [58, 59]. Testosterone substitution might not only not prevent CVD – a review revealed an increased risk of subsequent CVD [60]. However, most clinicians agree that men with endocrine hypogonadism and symptoms as reduced libido or loss of energy should be offered testosterone treatment unless there are contraindications.
11.10 Fatigue
Chronic fatigue (CF) is described as a subjective feeling of emotional, physical, and/or cognitive tiredness that is not relieved by rest, persisting for more than 6 months. The general male population in Norway previously reported a CF rate of 10 %, with a slight increase with age [61]. CF has repeatedly been reported to be associated with anxiety and depression in the literature, and associations with comorbidity, low educational level, not living in a relationship, being female, and increasing age have also been described [61, 62]. Significantly higher levels of C-reactive protein and interleukin-1 receptor antagonist have been reported to occur more frequent for TCSs with CF, indicating a possible association between CF and a low-grade inflammatory response [63]. Also, a significantly higher frequency of CF (16 %) was reported in a cross-sectional Norwegian study of long-term TCSs median 12 years after treatment for TC when compared with the age-matched Norwegian population [62]. Of note, the prevalence of CF increased from 15 to 27 % from one to two decades after treatment, respectively [64]. Two decades after TC treatment, the risk for CF was increased 5-fold for high levels of neuropathy and 2–3-fold for high levels of Raynaud’s phenomenon, physical inactivity, cardiac disease, and having testosterone levels in the lowest quartile. Furthermore, combination of these risk factors increased the likelihood of CF considerably.
11.11 Implications for Follow-Up
Risk factors for CVD like obesity, hypertension, hypercholesterolemia, and diabetes are more prevalent in TCS treated with cytotoxic treatment than in those treated with surgery only. However, this treatment burden of life-saving chemotherapy for metastatic TC has to be accepted. For these young men, it is essential to improve unfavorable lifestyle factors like obesity, hypertension, hypercholesterolemia, and possibly hypogonadism. Consequently, TCSs should be informed of the importance of maintaining a healthy lifestyle to reduce the risk of future CVD events. Preventive measures such as smoking cessation, keeping a healthy diet, and an active lifestyle may play an important role in reducing the potential risk of CVD from cytotoxic treatment in TCS [65]. Smoking cessation is also important in order to avoid a declining pulmonary function. Since the incidence of many cancers is related to an unhealthy lifestyle, promotion of a healthy lifestyle is probably important also in order to prevent SMN, although not tested in any randomized study among TCS. The diagnosis of cancer might represent a window of opportunity with regard to promoting lifestyle changes [66].
Awareness about the possibility of second cancers and CVD among TCS as well as their healthcare providers is important, and in case of symptoms, prompt diagnostic workup should be initiated.
11.12 Conclusions
Cure from cancer by chemotherapy comes at a prize in form of toxicities. The aim to keep this prize as low as possible dictates adherence to international guidelines. Whereas treatment of metastatic TC is quite homogenous worldwide, the role of adjuvant chemotherapy for stage I TC as opposed to active surveillance is controversial and depends, in light of excellent survival rates, on the potentially different long-term toxicities between both approaches. TC patients should be informed about acute and long-term toxicities before treatment and should be provided with a survivorship care plan after follow-up. An example of a one-page summary of TC treatment, inherent risks, and recommendations is available at www.swenoteca.org.
References
1.
Fossa SD, Aass N, Harvei S, Tretli S. Increased mortality rates in young and middle-aged patients with malignant germ cell tumours. Br J Cancer. 2004;90(3):607–12.CrossRefPubMedCentralPubMed
2.
3.
4.
5.
6.
7.
8.
van Leeuwen FE, Stiggelbout AM, Vandenbeltdusebout AW, Noyon R, Eliel MR, Vankerkhoff EHM, et al. second cancer risk following testicular cancer – a follow-Up-study of 1,909 patients. J Clin Oncol. 1993;11(3):415–24.PubMed
9.
10.
Horwich A, Fossa SD, Huddart R, Dearnaley DP, Stenning S, Aresu M, et al. Second cancer risk and mortality in men treated with radiotherapy for stage I seminoma. Br J Cancer. 2014;110(1):256–63.CrossRefPubMedCentralPubMed
11.
Andreassen KE, Grotmol T, Cvancarova MS, Johannesen TB, Fossa SD. Risk of metachronous contralateral testicular germ cell tumors: a population-based study of 7,102 Norwegian patients (1953–2007). Int J Cancer. 2011;129(12):2867–74.CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree