Warm autoimmune hemolytic anemia (AIHA) is defined as the destruction of circulating red blood cells (RBCs) in the setting of anti-RBC autoantibodies that optimally react at 37°C. The pathophysiology of disease involves phagocytosis of autoantibody-coated RBCs in the spleen and complement-mediated hemolysis. Thus far, treatment is aimed at decreasing autoantibody production with immunosuppression or reducing phagocytosis of affected cells in the spleen. The role of complement inhibitors in warm AIHA has not been explored. This article addresses the diagnosis, etiology, and treatment of warm AIHA and highlights the role of complement in disease pathology.
Key points
- •
Warm autoimmune hemolytic anemia (AIHA) is a rare disease, occurring in both idiopathic and secondary forms; common secondary etiologies include lymphoproliferative disorders, autoimmune disease, and drugs.
- •
Diagnosis involves the presence of markers of hemolysis and a positive direct Coombs test, which most commonly reveals red blood cell–bound immunoglobulin G antibodies with or without C3 complement.
- •
Erythrocyte destruction in warm AIHA is mediated by extravascular phagocytosis in the spleen and, in some cases, may involve complement-mediated mechanisms.
- •
Complement may exacerbate intravascular and extravascular hemolysis and is often a major contributor to severe forms of warm hemolytic crisis.
- •
First-line therapy includes corticosteroids followed by splenectomy or rituximab. The role of anticomplement agents in warm AIHA is unknown.
Introduction
Autoimmune hemolytic anemia (AIHA) is defined as the destruction of circulating red blood cells (RBCs) in the setting of anti-RBC autoantibodies. The in vivo and in vitro behavior of these autoantibodies allows for classification of AIHA into 3 forms: a warm type that causes agglutination of the blood at 37°C, a cold agglutinin that optimally reacts at 0°C to 5°C, and mixed-type disease that displays features of both. Warm AIHA is often suspected in the patient who develops hemolytic anemia with characteristic morphologic findings of microspherocytes on the peripheral smear. The disease occurs in both idiopathic and secondary settings, making investigation of potential underlying etiologies essential. The diagnosis ultimately requires confirmation with identification of an anti-RBC autoantibody by direct antiglobulin tests (DAT) and by the presence or absence of complement fractions, which also contribute to the pathogenesis of disease. Warm AIHA is most often associated with a positive DAT for RBC-bound immunoglobulin (Ig)G and/or C3.
History
The first cases of acquired warm hemolytic anemia were identified in the 1890s by Georges Hayem, who described a series of patients with the hallmark features of chronic jaundice, anemia, and splenomegaly. He noted that the type of jaundice was marked by a lack of biliary pigment, differentiating the condition from usual hepatic etiologies. However, despite the early initial recognition of the clinical presentation, the syndrome was not widely recognized until the mid-20th century. In 1940, Dameshek and Schwartz compiled previously published cases of acquired hemolytic jaundice, leading to the hypothesis that the disease was secondary to direct RBC lysis and that the severity of disease was related to the degree of hemolysis. Around the same time, interest in RBC immunobiology began to peak, and by 1945, the antiglobulin test was developed by an astute veterinarian, Robin Coombs. This essential blood-banking test was first used to identify pathogenic RBC antibodies in cases of hemolytic disease of the newborn, and both the direct and indirect antiglobulin tests now bear his name. The role of nonimmunoglobulin elements, such as complement, in the pathobiology of disease was not recognized until the 1950s.
Epidemiology
AIHA is known to be a rare disorder, although the non–disease-specific prevalence and incidence rates have not been widely reported. The incidence seems to be low at 0.8 to 3 cases per 100,000 person-years, resulting in an overall prevalence of about 17 cases per 100,000 individuals. Warm-reacting autoantibodies comprise the majority of cases of AIHA. Among cases of warm AIHA, primary or idiopathic disease occurs in about 50% of cases, with the remainder occurring in the setting of an underlying malignant, autoimmune, infectious, or drug triggers. For idiopathic warm AIHA, females are more commonly affected than males and are generally affected in their fourth or fifth decade. The demographic prevalence of secondary causes varies widely and is primarily driven by the underlying disease.
Introduction
Autoimmune hemolytic anemia (AIHA) is defined as the destruction of circulating red blood cells (RBCs) in the setting of anti-RBC autoantibodies. The in vivo and in vitro behavior of these autoantibodies allows for classification of AIHA into 3 forms: a warm type that causes agglutination of the blood at 37°C, a cold agglutinin that optimally reacts at 0°C to 5°C, and mixed-type disease that displays features of both. Warm AIHA is often suspected in the patient who develops hemolytic anemia with characteristic morphologic findings of microspherocytes on the peripheral smear. The disease occurs in both idiopathic and secondary settings, making investigation of potential underlying etiologies essential. The diagnosis ultimately requires confirmation with identification of an anti-RBC autoantibody by direct antiglobulin tests (DAT) and by the presence or absence of complement fractions, which also contribute to the pathogenesis of disease. Warm AIHA is most often associated with a positive DAT for RBC-bound immunoglobulin (Ig)G and/or C3.
History
The first cases of acquired warm hemolytic anemia were identified in the 1890s by Georges Hayem, who described a series of patients with the hallmark features of chronic jaundice, anemia, and splenomegaly. He noted that the type of jaundice was marked by a lack of biliary pigment, differentiating the condition from usual hepatic etiologies. However, despite the early initial recognition of the clinical presentation, the syndrome was not widely recognized until the mid-20th century. In 1940, Dameshek and Schwartz compiled previously published cases of acquired hemolytic jaundice, leading to the hypothesis that the disease was secondary to direct RBC lysis and that the severity of disease was related to the degree of hemolysis. Around the same time, interest in RBC immunobiology began to peak, and by 1945, the antiglobulin test was developed by an astute veterinarian, Robin Coombs. This essential blood-banking test was first used to identify pathogenic RBC antibodies in cases of hemolytic disease of the newborn, and both the direct and indirect antiglobulin tests now bear his name. The role of nonimmunoglobulin elements, such as complement, in the pathobiology of disease was not recognized until the 1950s.
Epidemiology
AIHA is known to be a rare disorder, although the non–disease-specific prevalence and incidence rates have not been widely reported. The incidence seems to be low at 0.8 to 3 cases per 100,000 person-years, resulting in an overall prevalence of about 17 cases per 100,000 individuals. Warm-reacting autoantibodies comprise the majority of cases of AIHA. Among cases of warm AIHA, primary or idiopathic disease occurs in about 50% of cases, with the remainder occurring in the setting of an underlying malignant, autoimmune, infectious, or drug triggers. For idiopathic warm AIHA, females are more commonly affected than males and are generally affected in their fourth or fifth decade. The demographic prevalence of secondary causes varies widely and is primarily driven by the underlying disease.
Diagnosis
Clinical Symptoms
AIHA is a clinicopathologic diagnosis that requires the presence of clinical features of hemolysis as well as serologic positivity for RBC-directed antibodies and/or complement fractions. The clinical spectrum of disease in warm AIHA is wide, with some patients presenting with mild, asymptomatic anemia and others presenting with a life-threatening hemolytic crisis. Common features include symptoms related to the degree of anemia, such as fatigue, weakness, pallor, and dyspnea on exertion, and symptoms related to hemolysis, such as jaundice, hemoglobinuria, or splenic fullness.
Laboratory Evaluation
Serologic diagnosis of warm AIHA requires confirmation of hemolytic anemia and pathologic anti-RBC immunoglobulins with or without RBC-bound complement. The hemolysis associated with warm AIHA is classically extravascular, or occurring in the spleen, although intravascular hemolysis is also common and may account for many of the fulminant cases of warm AIHA. Near universal laboratory features of hemolysis include low hemoglobin, elevated reticulocyte count, and elevated lactate dehydrogenase levels. Depleted haptoglobin levels and elevated indirect bilirubin may also been seen in a majority of cases. The anemia may often be macrocytic because of the degree of reticulocytosis. In a series of 69 cases of primary and secondary warm AIHA, the mean hematocrit at presentation was 25% with a mean reticulocyte percentage of 14% to 18%. The peripheral smear often demonstrates microspherocytes, which are thought to be a manifestation of the reduced surface-to-volume ratio caused by phagocytosis of immunoglobulin-coated RBCs in the spleen ( Fig. 1 ). Howell-Jolly bodies also may be seen after splenectomy.
The primary laboratory method for the diagnosis of AIHA is the direct antiglobulin (Coombs) test (DAT). The DAT is performed by adding a poly-specific antiglobulin reagent to a washed, suspended sample of the patient’s blood. Agglutination of the cells after centrifugation confirms that the antiglobulin has resulted in crosslinking of antibody-coated RBCs. A similar method is used for the detection of C3d-coated RBCs. An indirect antiglobulin test can also be used to detect unbound antibodies in the RBC eluate. In the indirect method, the serum and/or RBC eluate are incubated with donor RBCs. Agglutination after addition of antiglobulin suggests that circulating RBC-specific immunoglobulins in the patient’s serum have bound to the donor RBCs. This method can be used to differentiate autoantibodies from alloantibodies because autoantibodies tend to be panagglutinins (ie, they react with all panel RBCs), whereas alloantibodies are RBC antigen specific. The most common DAT findings in warm AIHA are +IgG (and rarely IgA or IgM) with or without +C3. The serum and indirect DAT findings will reveal a panagglutinating IgG.
Although positive DAT findings are suggestive of AIHA, the presence of serologic markers of AIHA in the absence of clinical hemolysis can occur in many settings. Approximately 0.1% of healthy blood donors and up to 8% of asymptomatic hospitalized patients may have a positive DAT. A positive DAT also occurs frequently in the setting of delayed transfusion reactions and after administration of intravenous immunoglobulin (IVIG). In contrast, a false-negative DAT is rare, but may occur in a small proportion (3%) of patients with AIHA, possibly secondary to low titer immunoglobulins, low-affinity autoantibodies, or the presence of warm IgA or IgM antibodies. In these cases, warm AIHA should be suspected if the smear demonstrates microspherocytes, the clinical presentation is typical of warm AIHA, or if the patient responds to empiric immunosuppressive therapy. Additional testing may detect the culprit autoantibody in DAT-negative AIHA; for example, IgA-specific antiglobulin can be used to diagnose IgA-mediated disease and high sensitivity assays, such as microcolumn DAT techniques or flow cytometry can be used to detect low-titer or low-affinity disease.
Although IVIG administration may lead to Coombs positivity in the absence of hemolysis, clinically significant reactions, including severe intravascular hemolysis, have been reported. These reactions are thought to occur in the setting of passive transfer of major and minor blood group antibodies, such that those with non-O blood groups are more susceptible to hemolytic reactions. Because each batch of IVIG contains different immunoglobulins, it is not easy to predict the risk of developing clinical hemolysis. Suggested approaches include cross-matching before IVIG infusion or monitoring laboratory parameters, including DAT and hemolysis serologies after infusion, so that further treatment can be withheld if signs of hemolysis are present.
Etiology
Autoantibody Response
The pathophysiology of autoantibody generation in AIHA is poorly understood but likely involves the interplay of multiple complex processes, including immune dysregulation and abnormal autoantigen response. The most common target for warm agglutinins are Rhesus (Rh) polypeptides on the RBC surface, and despite their association with clonal lymphoproliferative disorders, these warm autoantibodies are usually polyclonal. The underlying stimulus for autoantibody production in AIHA may involve molecular mimicry secondary to cross-reactivity of endogenous RBC and environmental antigens. Alternatively, abnormal processing of autoantigens in the setting of environmental factors may overcome self-tolerance. Immune dysfunction of B and T cells also likely contributes to autoimmunity in AIHA, because immunodeficiency states such infection with human immunodeficiency virus and lymphoproliferative neoplasms such as chronic lymphocytic leukemia (CLL), are often associated with warm AIHA and DAT positivity.
The mechanism of RBC destruction in the setting of warm autoantibodies involves the recognition of the Fc portion of the IgG molecule by tissue macrophages in the spleen. The degree of extravascular hemolysis depends the antibody titer, IgG subclass, and presence of bound complement, which can also be recognized and cleared by the spleen.
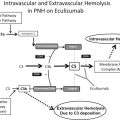
Role of Complement
Unlike in cold agglutinins disease, which is mediated exclusively by IgM, warm AIHA is only variably associated with directly bound complement because the most commonly involved autoantibody, IgG, does not universally fix complement. Nonetheless, many cases of IgG-mediated warm AIHA and nearly all cases of IgM-mediated warm agglutinins demonstrate a positive DAT for C3, which can result in intravascular hemolysis and potentiate ongoing extravascular phagocytosis. In IgG-mediated disease, the subclass of the pathologic antibody is relevant; IgG1 and IgG3 subclasses are associated with an increased ability to bind complement. Intravascular hemolysis is mediated by the cytotoxic effects of the membrane attack complex (C5b-C9), which is the end product of the complement cascade. The intravascular hemolysis related to complement can be robust and is present frequently in fulminant cases of warm AIHA. In fact, mortality rates in IgM-related warm autoimmune disease are high, likely owing to the degree of complement activation. Serum- and RBC-bound complement regulatory proteins, however, may mitigate the degree of intravascular hemolysis in many cases of warm AIHA, because C3 DAT positivity is often detected without evidence of intravascular RBC lysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree