CLINICAL PEARLS
Visual impairment affects 20% to 30% of persons aged 75 and older.
Cataracts and refractive error are common; both are correctable, and correction improves quality of life.
The dry form of age-related macular degeneration is common and antioxidant multivitamins may slow progression.
The wet form of macular degeneration is the most common cause of blindness in the elderly and may respond to laser surgery or antiangiogenesis inhibitors.
Glaucoma is the most common cause of blindness in the elderly African American population.
Screening for glaucoma should take place every 1 to 2 years after age 50.
Elevated intraocular pressure is not an absolute diagnostic criteria for glaucoma.
Therapies aim to reduce intraocular pressure, protecting the optic disc and preserving visual fields.
Visual impairment, defined as visual acuity <20/40, increases exponentially with age, such that 20% to 30% of the population at age 75 years is affected (see
Table 11.1 for a summary of the conditions that cause vision loss in the elderly). Blindness (visual acuity of 20/200 or worse) affects 2% of the population aged 75 years and older. Those aged 65 and older make up 12% of the US population, but they constitute 50% of the blind population. Cataract, age-related macular degeneration (ARMD), diabetic retinopathy, and glaucoma are the most common causes of blindness.
Among all office visits by older persons, 14% are to ophthalmologists, one of the highest rates among all specialty visits. Falls and car crashes, both associated with impaired vision, consume considerable medical resources. Impaired vision is linked to deterioration in the quality of life and in the activities of daily living of older persons.
The American Academy of Ophthalmology recommends a comprehensive eye examination every 1 to 2 years for
persons aged 65 and older. The U.S. Preventive Services Task Force recommends annual vision testing. One third of all new cases of blindness can be avoided with effective use of available ophthalmologic services.
REFRACTIVE ERROR AND CATARACT
The two leading causes of visual impairment worldwide are refractive error and cataract, for which eyeglasses and surgical cataract extraction, respectively, are mainstays of treatment. Despite the considerable successes of these therapeutic options, many people do not receive adequate treatment for these problems.
Refractive error may be categorized as emmetropia (neutral refraction), ametropia, or presbyopia. Three forms of ametropia exist: Myopia (nearsightedness), hyperopia (farsightedness), and astigmatism (distorted vision).
Typically, older patients demonstrate increasing hyperopia, unless a cataract is present, which can induce a myopic shift. Although contact lens and laser refractive surgery are available for myopic and hyperopic refractive errors, these alternative forms of treatment have traditionally been used by younger persons.
After the age of approximately 40, emmetropic persons begin to develop progressive presbyopia, impaired ability to focus on near objects that is caused by gradual hardening of the lens and decreased muscular effectiveness of the ciliary body. Reading glasses or bifocal eyeglasses may be prescribed.
Monovision with laser in situ keratomileusis (LASIK) is currently an option for presbyopic or pre-presbyopic patients seeking refractive surgery. This procedure corrects one eye for reading while the fellow eye is corrected for distance. Implantation of an anterior chamber multifocal intraocular lens after cataract surgery is another option for the correction of presbyopia. Finally, a new form of surgery under investigation uses an expansion of the sclera in the ciliary body region adjacent to the equator of the lens, which allows the ciliary body greater ability to change the anterior curvature of the lens as an accommodation to delay the onset of presbyopia.
Cataracts (vision reducing lens opacities) occur in 20% of persons aged 65 years and 50% aged 75 years and older. Cataracts may be associated with increased glare, decreased contrast sensitivity, and decreased visual acuity. Several risk factors have been reported: Decreased vitamin intake, light (ultraviolet B) exposure, smoking, alcohol use, long-term corticosteroid use, and diabetes mellitus. The most important risk factor is increased age.
1 Different types of cataracts present with distinct effects on visual acuity. The three main types of age-related cataracts are nuclear, cortical, and posterior subcapsular cataracts.
2 Often, patients have more than one type of cataract.
Nuclear cataracts appear as yellowing and sclerosis of the lens nucleus, best distinguished on slit lamp examination. In a primary care setting, the direct ophthalmoscope can be used from an arm’s length distance to judge for dimming of the red reflex caused by a central opacity in the lens. Nuclear cataracts are usually bilateral but exhibit varying degrees of asymmetry. They are slowly progressive and tend to cause greater impairment of distance vision than of near vision. Patients can present with a myopic shift in their refraction, permitting previously presbyopic individuals to read without reading glasses (“second sight”). Progressive yellowing of the lens causes decreased contrast sensitivity manifested by poor penetration of visible light onto the retina.
It is important to note that no degree of cataract will cause a Marcus Gunn pupil. Patients who present with more insidious onset of vision loss and a relative afferent pupillary defect warrant a more thorough ophthalmic examination to assess the health of the optic nerve and retina.
Cortical cataracts present early with formation of vacuoles and water clefts in the anterior and posterior lens cortex. These vacuoles can best be seen with a slit lamp on
retroillumination or by using a direct ophthalmoscope to obtain a red reflex from an arm’s length distance. As cortical changes progress, white wedge-shaped opacities, known as
cortical spokes, can be seen at the lens periphery, projecting to the center of the lens. On retroillumination using a direct ophthalmoscope, the cortical spokes appear as shadows obscuring the red reflex. In more advanced cases, the peripheral cortical opacities can coalesce to involve the entire lens cortex, giving the lens a diffuse white and opaque appearance. Cortical cataracts are usually bilateral but tend to be more asymmetric than nuclear cataracts. They have greater variability in their rate of progression, and their effect on visual acuity depends on the location of the cortical opacity. Glare is commonly associated with cortical cataracts, as manifested by decreased vision when staring at a light source such as a car headlight. Less commonly, cortical cataracts can be the source of monocular diplopia.
Posterior subcapsular cataracts first present with a subtle sheen in the posterior cortical layers visible only by slit lamp examination. More advanced stages exhibit granular and plaque-like opacities on the posterior subcapsular cortex, which are best seen with retroillumination using a slit lamp or a direct ophthalmoscope. This type of cataract is more often seen in younger patients than in those with advanced nuclear or cortical cataracts. In addition to its age-related etiologies, it can also be associated with systemic or topical corticosteroid use, history of ocular inflammation, trauma, or exposure to ionizing radiation. Patients with posterior subcapsular cataracts often complain of glare and poor vision in bright settings. This is secondary to constriction of the pupil, which narrows the visual axis to that provided by the central cataractous lens.
When evaluating a patient with cataracts, a physician should objectively measure visual acuity. Distance vision using a Snellen chart as well as near vision using a reading card should both be checked. In a patient with cataracts, pinhole distance visual acuity is often better than that obtained with best refractive correction. In cases of posterior subcapsular and cortical cataracts, distance vision should be checked with the application of a light source directed at the patient’s eyes to measure visual impairment caused by glare. The next step is correlation of the degree of media opacity with the degree of visual impairment.
In addition to careful examination of the macula and the optic nerve, a careful history detailing the time course of vision loss and the patient’s baseline visual function is important. The main objective of this step is determining that the patient’s eye is healthy enough to expect improved visual function after cataract surgery. Finally, consideration must be given to the patient’s overall health and ability to undergo surgery, in addition to his/her ability to participate in postoperative care.
Cataract extraction is one of the most successful surgeries in medicine (90% of patients achieve vision of 20/40 or better). Approximately 1.5 million cataract procedures are performed each year in the United States alone. In inner cities and underdeveloped countries such as India, the demand for surgery has surpassed available resources.
Cataract extraction is safe and can be completed in <15 minutes under local or topical anesthesia. The surgery involves the sonographic breakdown and aspiration of the lens (phacoemulsification) through a 3 to 4 mm limbal (corneoscleral junction) incision. An artificial implant (intraocular lens) is placed in the capsular bag that is the only remnant of the native lens retained. A secondary laser procedure (capsulotomy) may be necessary to ablate subsequent capsular opacification that develops in 15% of patients. Typically, patients will require spectacles only for reading postoperatively, although the U.S. Food and Drug Administration (FDA) has recently approved the use of multifocal lens implants that may negate the use of glasses altogether.
AGE-RELATED MACULAR DEGENERATION
ARMD is the most common cause of blindness in older persons throughout the developed world. Approximately 1 in 27 Americans (10 million people) have reduced vision due to ARMD. Increased age is the most important risk factor, although a genetic predisposition also contributes. Other risk factors include smoking and hypertension. Fairskinned persons are at greater risk of developing this disease than those who are black, in whom pigment may serve as a protective element.
Severe vision loss or central visual blindness is typically caused by the wet form of ARMD, defined by the presence of a choroidal neovascular membrane (CNVM). Less commonly, geographic atrophy of the retinal pigment epithelium (RPE) within the macular region noted in the late stage of the dry form of ARMD may explain the severe vision loss. The natural history of CNVM is progressive subfoveal growth with leakage, bleeding, and eventual fibrotic scarring, leading to central blindness.
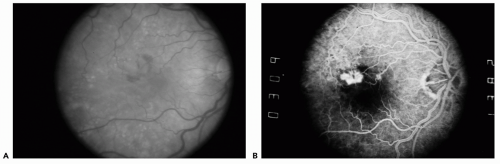
The most common form of ARMD is the dry type characterized by drusen, which appear clinically as dull yellow deposits located in the submacular compartment deep to the retinal vessels (see
Fig. 11.1). Drusen are classified by their number, size, and confluence. The presence of CNVM in the fellow eye and numerous, large confluent drusen represent the greatest risk for progression to the wet form of ARMD. Associated with the presence of drusen, RPE mottling or atrophy is a hallmark of the dry form of ARMD. Clinically, mottling of the RPE appears as a focal or geographic area of pigment clumping and depigmentation in the retina. Geographic atrophy is denoted by a large central and circumscribed area of RPE atrophy that exposes the underlying choroidal vessels. This type of atrophy represents the late stage of ARMD and can cause central blindness although CNVM is absent and the macula is dry.
It is important to identify the clinical markers for CNVM, which include subretinal heme, subretinal fluid, and the presence of a gray green membrane or pigment ring. On examination, subretinal heme can be differentiated from intraretinal heme by its deeper location with regard to the retinal vessels (
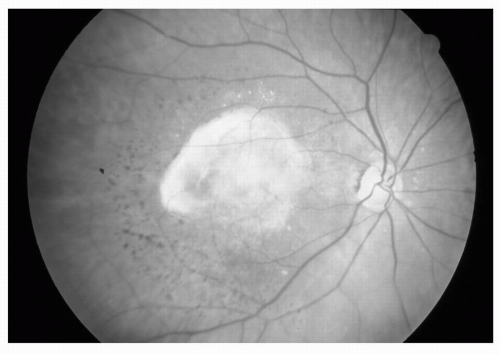
Fig. 11.1). The end stage of the wet form of ARMD shows cicatrization or fibrotic proliferation known as a
disciform scar, which clinically appears as an elevated white lesion in the macula (see
Fig. 11.2).
In a primary care setting, patients older than age 50 with no prior diagnosis of ARMD who present with clinical markers of dry form ARMD should be referred to an ophthalmologist for routine monitoring on the basis of the stage of the disease. Early dry form ARMD warrants annual funduscopic examination, while the later stages of dry form ARMD require biannual examination.
The Age-Related Eye Disease Study (AREDS) found that the risk for the development of CNVM could be reduced by 25% when patients with high-risk drusen (numerous and large) are treated with high-dose multivitamin supplementation.
3 A combination multivitamin supplement containing
β-carotene 25,000 IU, vitamin E 400 IU, vitamin C 500 mg, and zinc 80 mg is available as an over-the-counter preparation (Bausch and Lomb, Ocuvite PreserVision). This vitamin therapy, however, is contraindicated in smokers because of the increased risk of lung cancer due to the high-dose
β-carotene component. Patients with the dry form of ARMD should be provided with an Amsler grid, a good home-monitoring tool, to regularly test for metamorphopsia (Evidence Level C). Those who develop sudden distortion or vision loss, signaling the development of CNVM, require urgent ophthalmological evaluation.
Laser therapy for CNVM has been beneficial, but only in circumstances when membranes are well defined (i.e., the margins of the lesion are clearly delineated on fluorescein angiography) and are extra- or juxtafoveal. The benefits of this therapy are limited, as 50% to 75% of lesions recur after laser therapy. Photodynamic therapy using porphyrin-derived dyes has been shown to be useful in those patients with subfoveal CNVM in whom conventional laser therapy will destroy the fovea. According to the Treatment of ARMD with Photodynamic therapy (TAP) study, 43% of patients receiving photodynamic therapy sustained moderate visual loss (>15 letters from baseline) after 1 year of follow-up versus 57% of patients not receiving the photoactivated dye laser treatment. Moreover, 16% of those receiving photoactivated dye laser treatment sustained an improvement in visual acuity while 7% showed improvement in the placebo group. These benefits continued after 2 years of follow-up but required as many as five sessions of photodynamic therapy.
4 Subfoveal membranes that were predominantly classical appeared to have the greatest response to photodynamic therapy, although a later study has shown marginal benefit for the treatment of small, purely occult subfoveal CNVM as well. Overall, the benefits of photodynamic therapy are not overwhelming, but photodynamic therapy is a relatively safe option for delaying vision loss in the wet form of ARMD.
Intravitreal injections of inhibitors to vascular endothelial growth factor (VEGF) have been validated as an adjuvant therapy for the wet form of ARMD (Evidence Level B). FDA approval has recently been obtained for the treatment of wet form ARMD with serial intravitreal injections of pegaptanib sodium (Macugen), an aptamer which blocks the 125 isoform of VEGF. The FDA approval was based on findings from two pivotal phase 2/3 randomized multicenter double-masked clinical trials involving approximately 1,200 patients with all subtypes of neovascular ARMD.
5 Results showed that among patients receiving 0.3 mg of Macugen, 70% lost less than three lines of vision compared with 55% of patients receiving control treatment (
p < 0.0001), a 27% increased chance of stabilization after the first year. Macugen also helped limit progression to legal blindness by 50% compared to controls. Two-year clinical data from the studies demonstrated a continued treatment benefit with Macugen.
Other VEGF inhibitor therapies include the intravitreal injection of ranibizumab (Lucentis), an antibody fragment that inhibits all isoforms of VEGF, and its intravenous counterpart bevacizumab (Avastin), each of which is presently being investigated by the FDA and holds great promise for the treatment of wet form ARMD.
Another treatment modality for subfoveal CNV currently under investigation, anecortave acetate, targets proteases required for vascular endothelial cell migration. Anecortave acetate, an angiostatic steroid, is administered as a posterior juxtascleral depot application at 6-month intervals. Recent 12-month clinical outcomes have shown the drug to be efficacious in maintaining vision, preventing severe vision loss, and inhibiting subfoveal CNV lesion growth.
6Unfortunately, laser therapies and intraocular injections do not exist for the atrophic form of ARMD. RPE and photoreceptor transplantation with or without stem cells have been extensively investigated in vitro and in animal models but have not reached the clinical realm.
Numerous genetic conditions resulting in premature macular degeneration exist, for which the mutated sequence has been cloned and the protein product isolated. Mutations in ABCA4 (adenosine triphosphate [ATP]-binding cassette protein of the retina), a photoreceptor protein involved in molecular transport and exchange, leads to the development of Stargardt disease, in which macular drusenoid flecks and atrophy associated with central visual loss develop by the second or third decade of life. Stargardt macular dystrophy has been found to have perhaps some association with the dry or atrophic form of ARMD. Certain populations of patients with ARMD have been found to have an increased incidence of the heterozygous form of this mutation, but the data have been conflicting. Sorsby fundus dystrophy is associated with many of the features of ARMD. Visual loss, however, typically develops before the age of 50. The affected gene has been sequenced and the mutated protein, involved in extracellular remodeling, is TIMP-3 (tissue-inhibitor of metalloprotease-3). However, genetic studies have yet to uncover an association of this mutation with ARMD.
Most recently, much excitement has been generated by published studies from three separate centers that have uncovered as high as a 50% association of the high-risk forms of ARMD with a mutation in the complement factor
H gene. In individuals homozygous for the risk allele, the likelihood of ARMD was found to be increased by a factor of 7.4. The complement factor
H gene is located on chromosome 1 in a region that has been repeatedly linked to ARMD in family based studies,
7 and the protein is known to be a regulator of complement activation and may implicate inflammation in the progression of ARMD.