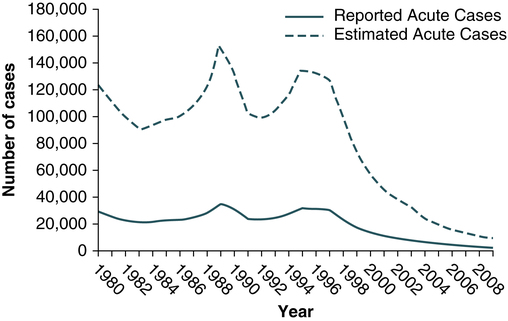
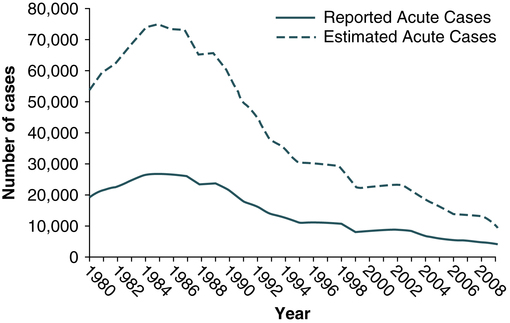
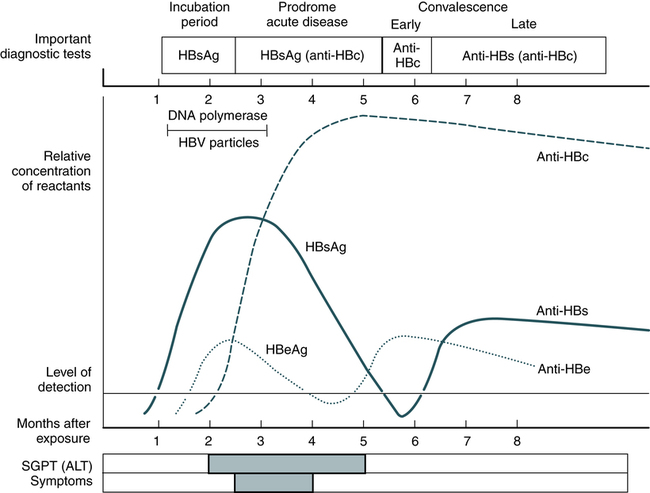
At the conclusion of this chapter, the reader should be able to: • Identify and describe the characteristics of the various forms of primary infectious hepatitis, including laboratory assays. • Compare the etiology, epidemiology, signs and symptoms, laboratory evaluation, and prevention of the various types of hepatitis. • Analyze case studies related to the immune response various forms of hepatitis. • Correctly answer case study related multiple choice questions. • Be prepared to participate in a discussion of critical thinking questions. • Describe the principle, results, and limitations of the rapid HCV test. Primary hepatitis viruses account for approximately 95% of the cases of hepatitis. These viruses are classified as primary hepatitis viruses because they attack primarily the liver and have little direct effect on other organ systems. The secondary viruses involve the liver secondarily in the course of systemic infection of another body system. The viruses for hepatitis types A, B, C, D, E, and GB virus C, as well as secondary viruses (e.g., EBV, CMV), have been isolated and identified (Table 23-1). Table 23-1 Characteristics of Viral Hepatitis As a clinical disease, hepatitis can occur in acute or chronic forms. The signs and symptoms of hepatitis are extremely variable. It can be mild, transient, and completely asymptomatic or it can be severe, prolonged, and ultimately fatal. Many fatalities are attributed to hepatocellular carcinoma in which hepatitis viruses B and C are the primary causes. The course of viral hepatitis can take one of four forms—acute, fulminant acute, subclinical without jaundice, and chronic (Table 23-2). Table 23-2 HAV is a small, RNA-containing picornavirus and the only hepatitis virus that has been successfully grown in culture (Fig. 23-1, A). The structure is a simple nonenveloped virus with a nucleocapsid designated as the hepatitis A (HA) antigen (HA Ag). Inside the capsid is a single molecule of single-stranded ribonucleic acid (RNA). The RNA has a positive polarity and proteins are translated directly from the RNA. Replication of HAV appears to be limited to the cytoplasm of the hepatocyte. Hepatitis A virus was formerly called infectious hepatitis or short-incubation hepatitis. In developing countries, hepatitis A is primarily a disease of young children; the prevalence of infection, as measured by the presence of antibody (immunoglobulin G [IgG] anti-HAV), approaches 100% at or shortly after 5 years of age. The national rate of hepatitis A has declined steadily since the last peak in 1995 (Fig. 23-2). After asymptomatic infection and underreporting were taken into account, an estimated 21,000 new infections occurred in 2009 (the last year for which statistics were available at the time of publication). A safe, highly immunogenic, formalin-inactivated, single-dose vaccine is available to prevent HAV infection (Box 23-1). HAV vaccine should be targeted at high-risk groups (e.g., staff in child care centers; food handlers; international travelers, including military personnel; homosexual men; institutionalized patients). HBV is the classic example of a virus acquired through blood transfusion. It serves as a model when transfusion-transmitted viral infections are considered (see Fig. 23-1, B). 2. A structural nucleocapsid core protein—hepatitis B core antigen (HBcAg) 3. A soluble nucleocapsid protein—hepatitis B e antigen (HBeAg) HBV relies on a retroviral replication strategy (reverse transcription from RNA to DNA). Eradication of HBV infection is rendered difficult because stable, long-enduring, covalently closed circular DNA (cccDNA) becomes established in hepatocyte nuclei and HBV DNA become integrated into the host genome (Fig. 23-3). Hepatitis B infection has been referred to as long-incubation hepatitis. In 2009 (the last year for which statistics were available at the time of publication), a total of 3371 acute symptomatic cases and 38,000 estimated total new infections of hepatitis B were reported in the United States. The overall incidence was the lowest ever recorded and represents a decline of 81% since 1990 (Fig. 23-4). The incidence of HBV infection caused by blood transfusion is increasingly rare in developed countries. Transfusion-acquired HBV has been severely reduced because high-risk donor groups (e.g., paid donors, prison inmates, military recruits) have been eliminated as major sources of donated blood and because specific serologic screening procedures have been instituted. This shift to an all-volunteer donor supply probably accounts for a 50% to 60% reduction of transfusion-related hepatitis. The overall incidence of HBV is high among patients who have received multiple transfusions or blood components prepared from multiple-donor plasma pools, hemodialysis patients, drug addicts, and medical personnel (see Table 23-1). • Homosexual men with multiple partners • Household contacts and sexual partners of HBV carriers • Infants born to HBV-infected mothers • Patients and staff in custodial institutions for developmentally disabled persons • Recipients of certain plasma-derived products, including patients with congenital coagulation defects • Health care and public safety workers who may be in contact with infected blood Laboratory diagnosis (Fig. 23-5) and monitoring of acute and chronic HBV infections involve the use of several of the following tests (Tables 23-3 and 23-4): Table 23-3 Serologic Markers for Hepatitis B Virus (HBV) Infection −, Negative; +, positive; ±, questionable. Adapted from Hoofnagle JH: Type A and type B hepatitis, Lab Med 14(11):713 1983. Table 23-4 Interpretation of Hepatitis B Panel 1.Might be recovering from acute HBV infection. 2.Might be distantly immune and test not sensitive enough to detect very low level of anti-HBs in serum. 3.Might be susceptible with a false-positive anti-HBc. 4.Might be undetectable level of HBsAg present in the serum and the person is actually chronically infected. 1. Hepatitis B surface antigen (HBsAg) 2. Hepatitis B e antigen (HBeAg) 3. Hepatitis B core antibody, total or IgM (anti-HBc) 4. Hepatitis B e antibody (anti-HBe) 5. Hepatitis B surface antibody (anti-HBs) 6. Hepatitis B viral DNA by polymerase chain reaction (PCR, qualitative and quantitative) The titer of HBsAg rises and generally peaks at or shortly after the onset of elevated liver serum enzyme levels (e.g., ALT, SGPT). Clinical improvement of the patient’s condition and a decrease in serum enzyme concentrations are paralleled by a fall in the titer of HBsAg, which subsequently disappears. There is variability in the duration of HBsAg positivity and in the relationship between clinical recovery and the disappearance of HBsAg (Fig. 23-6). About 5% of positive HBsAg values are false-positive results.
Viral Hepatitis
General Characteristics of Hepatitis
Incidence
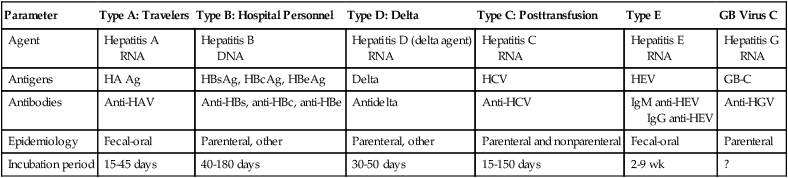
Parameter
Type A: Travelers
Type B: Hospital Personnel
Type D: Delta
Type C: Posttransfusion
Type E
GB Virus C
Agent
Hepatitis A
RNA
Hepatitis B
DNA
Hepatitis D (delta agent)
RNA
Hepatitis C
RNA
Hepatitis E
RNA
Hepatitis G
RNA
Antigens
HA Ag
HBsAg, HBcAg, HBeAg
Delta
HCV
HEV
GB-C
Antibodies
Anti-HAV
Anti-HBs, anti-HBc, anti-HBe
Antidelta
Anti-HCV
IgM anti-HEV
IgG anti-HEV
Anti-HGV
Epidemiology
Fecal-oral
Parenteral, other
Parenteral, other
Parenteral and nonparenteral
Fecal-oral
Parenteral
Incubation period
15-45 days
40-180 days
30-50 days
15-150 days
2-9 wk
?
Signs and Symptoms
Form
Characteristics
Acute hepatitis
Typical form with associated jaundice.
Four phases—incubation, preicteric, icteric, and convalescence
Incubation period, from time of exposure and first day of symptoms, ranges from few days to many months
Average length of time is 75 days (range, 40-180) in hepatitis B virus (HBV) infection
Fulminant acute hepatitis
Rare form of hepatitis associated with hepatic failure
Subclinical hepatitis without jaundice
Probably accounts for persons with demonstrable antibodies in their serum but no reported history of hepatitis
Chronic hepatitis
Accompanied by hepatic inflammation and necrosis that lasts for at least 6 mo
Occurs in about 10% of patients with HBV infection
Hepatitis A
Etiology
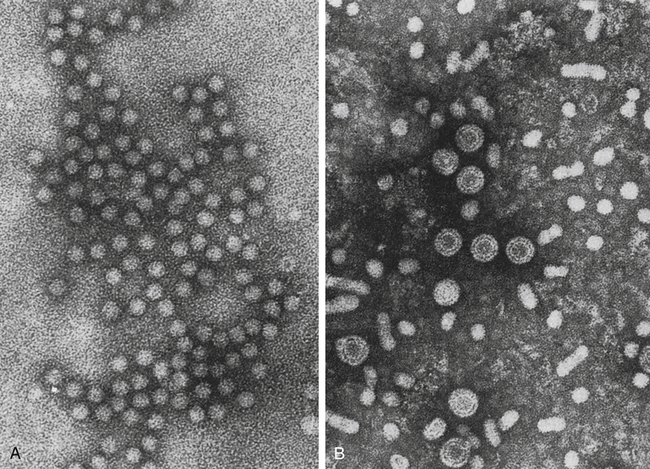
A, Hepatitis A virus (HAV). B, Hepatitis B virus (HBV). Note Dane particles. (From Katz SL, Gershon AA, Wilfert CM, Krugman S, editors: Infectious diseases of children, ed 9, St Louis, 1992, Mosby.)
Epidemiology
Prevention and Treatment
Hepatitis B
Etiology
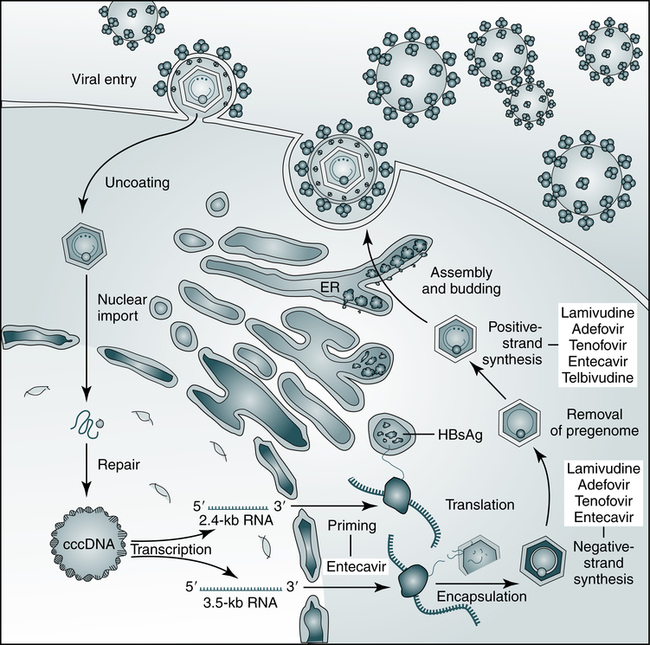
The hepatitis B virus (HBV) establishes covalently closed circular DNA (cccDNA) as a durable miniature chromosome in the host nucleus and relies on a retroviral strategy of reverse transcription from RNA to negative-strand DNA. The steps of HBV replication targeted by nucleoside and nucleotide analogues that are used to treat chronic HBV infection are shown. ER, Endoplasmic reticulum; HBsAg, hepatitis B surface antigen. (From Dienstag JL: Hepatitis B virus infection, N Engl J Med 359:1486–1500, 2008.)
Epidemiology
Laboratory Assays
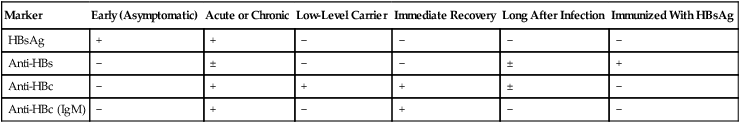
Marker
Early (Asymptomatic)
Acute or Chronic
Low-Level Carrier
Immediate Recovery
Long After Infection
Immunized With HBsAg
HBsAg
+
+
−
−
−
−
Anti-HBs
−
±
−
−
±
+
Anti-HBc
−
+
+
+
±
−
Anti-HBc (IgM)
−
+
−
+
−
−
Tests
Results
Interpretation
HBsAg
Anti-HBc
Anti-HBs
Negative
Negative
Negative
Susceptible
HBsAg
Anti-HBc
Anti-HBs
Negative
Positive
Positive
Immune because of natural infection
HBsAg
Anti-HBc
Anti-HBs
Negative
Negative
Positive
Immune because of hepatitis B vaccination
Acutely infected
HBsAg
Anti-HBc
IgM anti-HBc
Anti-HBs
Positive
Positive
Negative
Negative
Chronically infected
HBsAg
Anti-HBc
Anti-HBs
Negative
Positive
Negative
Four interpretations possible∗
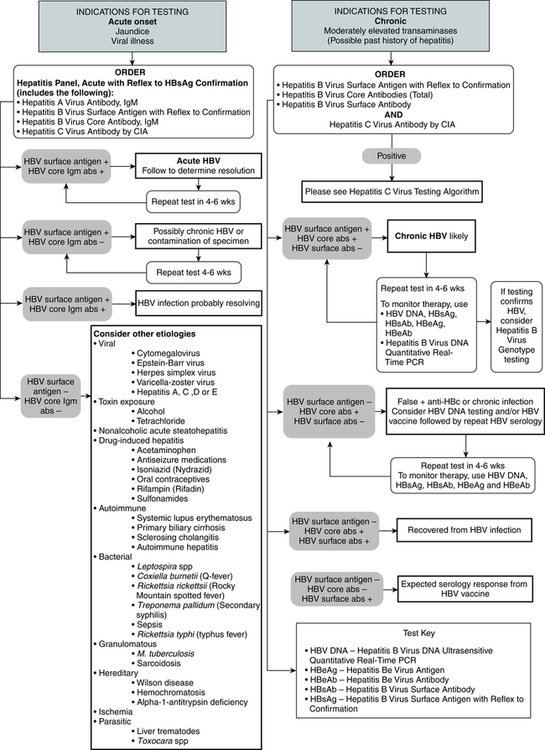
Hepatitis B Surface Antigen
Viral Hepatitis