and Winfried G. Rossmanith2
(1)
Lenzkirch, Germany
(2)
Ettlingen, Germany
4.1 Translation
4.1.3 Splicing
4.1.4 RNA Cap
4.1.6 Docking to Ribosomes
4.2.3 Protein Complexes
4.2.4 Glycosylation
4.2.5 Prohormone Convertases
4.2.7 Chopping the C-Terminus
4.2.10 Esterification of Ghrelin
4.3.3 Neuropeptide Y
4.3.4 Agouti-Related Protein
4.3.5 Somatostatin
4.3.6 Substance P
4.3.7 Proopiomelanocortin
4.3.8 Ghrelin
4.3.9 Kisspeptin
4.3.10 Galanin
4.3.12 Orexins
4.4.1 POMC
4.4.2 TSH
4.4.3 LH, FSH, CG
4.4.4 Growth Hormone
4.4.5 Prolactin
4.5.1 Somatolactin
4.6.1 Introduction
4.6.2 Structure and Genes
4.6.3 Physiology of Oxytocin
4.6.5 Phylogeny
4.7.1 Insulin
4.7.2 Glucagon
4.8.1 Leptin
4.8.2 Ghrelin
4.9.1 Introduction
4.9.2 Structure and Genes
4.9.3 Physiology
4.9.4 Phylogeny
4.10.1 Gastrin
4.10.2 Cholecystokinin
4.10.3 Secretin
4.10.4 VIP
4.10.5 GIP
4.10.6 PNP, NPY, PYY
4.11.1 Endorphins and Enkephalins
4.12.1 Activin/Inhibin
4.12.2 Follistatin
4.12.3 Antimüllerian Hormone
4.13.1 Introduction
4.13.2 Structure and Genes
4.13.3 Physiology
4.13.4 Phylogeny
4.14.1 Introduction
4.14.2 Structure and Genes
4.14.3 Physiology
4.14.4 Phylogeny
4.15.1 Parathormone
4.15.2 Stanniocalcin
4.15.3 Erythropoietin
Readers familiar with how proteins are made may skip the following section. For those who are not familiar, we provide a short introduction to this process from which all life has arisen. The mechanism of forming structures from the genetic blueprint is obviously as old as life itself because it is common to all forms of life on Earth.
4.1 Translation
4.1.1 Reading Genetic Information: Transcription
Genetic information if encoded in the chromosome by means of the sequence of four bases—adenine , cytosine , guanine , and thymine —in the double strand of deoxyribonucleic acid genetic information is coded for in the chromosomes. This information is transcribed into a single-stranded ribonucleic acid when a gene is activated. In the case of bacterial, viral, or many yeast genes, the RNA is directly coupled to ribosomes with whose help single amino acids are added to a protein sequence according to the code in the DNA.
4.1.2 Coding and Other Sequences
In eukaryotic cells the coding information on the DNA double strand is interspersed with noncoding chromosomal regions, which will never be used for protein synthesis. The coding sequences are called exons, those without coding information, introns.
4.1.3 Splicing
The primary RNA transcript still contains exons and introns. By a process called splicing the introns are removed. Splicing is performed using enzymatically active RNAs and proteins. These proteins are called splicing factors.
Many RNAs can be spliced to different products, alternative splicing; for obtaining differentially spliced RNA the just-mentioned splicing factors are responsible that are found in a cell-type-specific manner. Thirty different splicing factors have been found; their regulation is not yet well understood.
4.1.4 RNA Cap
Eukaryotic RNA has on its 5′ end an additional structure, the so-called RNA cap that reduces the RNA degradation in the cytoplasm.
4.1.5 Nuclear Export of Messenger RNA
RNA when spliced and capped is called messenger RNA (mRNA) . This mRNA is exported through the nuclear membrane with the help of transfer proteins and thus reaches the cytosol.
4.1.6 Docking to Ribosomes
In the cytosol two ribosomal subunits aggregate with the mRNA. Transfer RNA will load the amino acids into the ribosomes that will then be added to the protein sequence according to the genetic code. This process is called translation.
4.1.7 Translational Termination
A termination signal within the RNA sequence lets the ribosomal subunits fall off the mRNA. mRNA and ribosomes can be reused.
4.1.8 Membrane and Secretory Proteins
In the case of secreted proteins or membrane proteins this general translation pathway is extended. During translation membrane proteins are integrated into membranes although secretory proteins are not translated into the cytosol, but into special, membrane-sealed, cellular compartments, from where the secreted proteins, for example, hormones, are finally secreted.
These compartment are vesicles of the ER where synthesis of membrane proteins and secretory cell products takes place. These vesicles themselves are enclosed with a double membrane like the cell membrane. Other cellular compartments with separate double membranes are the eukaryotic nucleus, prokaryotes (i.e., bacteria or blue algae do not possess a nucleus), mitochondria, where energy is gained from sugars, and the Golgi apparatus, where protein maturation occurs. Secretory granules that contain the mature hormones ready for secretion are also separated from the cytosol by a double membrane.
Later, we demonstrate that some proteins of the steroid synthetic pathway are localized to mitochondria, some are found in the ER, and others stay in the cytosol. There exists a topological separation of different enzymatic functions of steroid-forming cascades.
4.2 Posttranslational Modification: Hormone Maturation
Precursors of protein/peptide hormones are formed at the membrane of the ER and they are translocated through this membrane into the ER vesicles. Therein and in other matured vesicles hormone maturation will occur.
4.2.1 Removal of the Signal Peptide
The first 22–30 amino acids of a precursor protein that is formed at the ER membrane are called signal peptides. Once the growing polypeptide chain has reached the interior of the ER the enzyme signal peptidase cleaves off this signal peptide, a process that is performed for membrane and secretory proteins.
4.2.2 Folding and Disulfide Bridges
The growing polypeptide chain moves through the ER pore as a linear strand. Within the ER this strand is folded into the three-dimensional structure characteristic for any protein. Folding is achieved with the help of chaperones, for example, heat shock proteins.
The three-dimensional protein structure resulting from folding contains mainly helices and 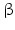 sheets. Other areas exist in an unordered form. Hydrogen bonds are essential structural elements for the maintenance of a given three-dimensional structure, as well as ionic and nonionic interactions between the amino acids of an individual protein. The use of supercomputers has not yet made it possible to create a general algorithm for protein folding: folding prediction has only been possible with varying success.
sheets. Other areas exist in an unordered form. Hydrogen bonds are essential structural elements for the maintenance of a given three-dimensional structure, as well as ionic and nonionic interactions between the amino acids of an individual protein. The use of supercomputers has not yet made it possible to create a general algorithm for protein folding: folding prediction has only been possible with varying success.
 sheets. Other areas exist in an unordered form. Hydrogen bonds are essential structural elements for the maintenance of a given three-dimensional structure, as well as ionic and nonionic interactions between the amino acids of an individual protein. The use of supercomputers has not yet made it possible to create a general algorithm for protein folding: folding prediction has only been possible with varying success.
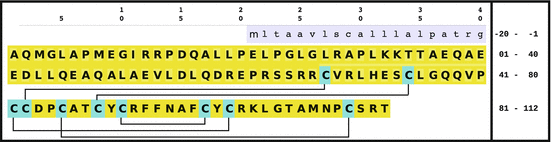
sheets. Other areas exist in an unordered form. Hydrogen bonds are essential structural elements for the maintenance of a given three-dimensional structure, as well as ionic and nonionic interactions between the amino acids of an individual protein. The use of supercomputers has not yet made it possible to create a general algorithm for protein folding: folding prediction has only been possible with varying success.Coupled with folding is the generation of intramolecular disulfide bonds thereby covalently linking two cysteine residues. These disulfide bonds together with the interactions just mentioned determine the three-dimensional protein structure. The glycoprotein hormones such as thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), choriogonadotropin (CG) or nerve growth factor, as well as the insect hormone bursicon, form a special cysteine knot (Fig. 4.1); two pairs of disulfide bonds with short amino acid sequences between adjacent cysteines form a belt. The third disulfide bond is directed through this belt (Fig. 4.1). Modifications of this knot structure render the protein nonfunctional. The proper formation of the cysteine knot is indispensable for these hormones. It appears almost self-evident that this structure had been conserved during evolution whereas other amino acids were exchanged. The distances between two cysteine residues and thus the chain length in between remained constant during vertebrate evolution, which gives a clue for the conservation of the functional properties, too.
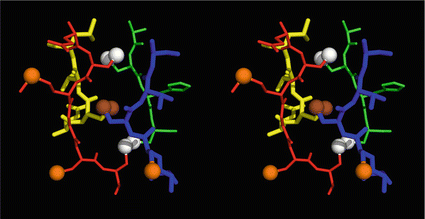

Fig. 4.1
Stereo view of the cysteine knot of the gonadotropin  -chain: two disulfide bonds (white: sulfur atoms) between Cys28 (red chain, amino acids 27–32) and cys82 (green chain, amino acids 81–84), and between Cys32 (red) to Cys84 (green) form a ring through which reaches the third disulfide bond between Cys10 (yellow chain, amino acids 9–12) and Cys60 (blue chain, amino acids 58–62) (brown: sulfur atoms) (Source: GenBank 1HRP and PyMOL)
-chain: two disulfide bonds (white: sulfur atoms) between Cys28 (red chain, amino acids 27–32) and cys82 (green chain, amino acids 81–84), and between Cys32 (red) to Cys84 (green) form a ring through which reaches the third disulfide bond between Cys10 (yellow chain, amino acids 9–12) and Cys60 (blue chain, amino acids 58–62) (brown: sulfur atoms) (Source: GenBank 1HRP and PyMOL)
 -chain: two disulfide bonds (white: sulfur atoms) between Cys28 (red chain, amino acids 27–32) and cys82 (green chain, amino acids 81–84), and between Cys32 (red) to Cys84 (green) form a ring through which reaches the third disulfide bond between Cys10 (yellow chain, amino acids 9–12) and Cys60 (blue chain, amino acids 58–62) (brown: sulfur atoms) (Source: GenBank 1HRP and PyMOL)
-chain: two disulfide bonds (white: sulfur atoms) between Cys28 (red chain, amino acids 27–32) and cys82 (green chain, amino acids 81–84), and between Cys32 (red) to Cys84 (green) form a ring through which reaches the third disulfide bond between Cys10 (yellow chain, amino acids 9–12) and Cys60 (blue chain, amino acids 58–62) (brown: sulfur atoms) (Source: GenBank 1HRP and PyMOL)4.2.3 Protein Complexes
The next step during hormone formation is the aggregation of identical or different polypeptides to larger complexes. This is a general feature not only of hormones, but also of many other proteins.
Within hormones the glycoprotein hormones are complexes of two different polypeptides. The first chain, the 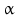 chain is the common chain of four different glycoprotein hormones, and the
chain is the common chain of four different glycoprotein hormones, and the 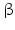 chain is characteristic for the four hormones: LH, FSH, TSH, and CG. Singular
chain is characteristic for the four hormones: LH, FSH, TSH, and CG. Singular 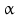 or
or 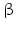 chains are nonfunctional. The complex of both chains is necessary to give rise to the proper structure that triggers the hormone receptor on the target cell.
chains are nonfunctional. The complex of both chains is necessary to give rise to the proper structure that triggers the hormone receptor on the target cell.
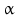 chain is the common chain of four different glycoprotein hormones, and the
chain is the common chain of four different glycoprotein hormones, and the 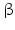 chain is characteristic for the four hormones: LH, FSH, TSH, and CG. Singular
chain is characteristic for the four hormones: LH, FSH, TSH, and CG. Singular 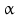 or
or 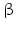 chains are nonfunctional. The complex of both chains is necessary to give rise to the proper structure that triggers the hormone receptor on the target cell.
chains are nonfunctional. The complex of both chains is necessary to give rise to the proper structure that triggers the hormone receptor on the target cell.Oxytocin has equally been found in a complex with other peptides, the neurophysins. Whereas 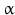 -glycoprotein and
-glycoprotein and 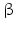 -glycoprotein hormone chains are transcribed from different genes, the oxytocin and the neurophysins are coded for in the same gene and transcribed into a single protein which is then processed during hormone maturation, however, the separated peptides stay together in a complex. At the final stage in the secretory granule, the mature oxytocin is no longer complexed to neurophysins.
-glycoprotein hormone chains are transcribed from different genes, the oxytocin and the neurophysins are coded for in the same gene and transcribed into a single protein which is then processed during hormone maturation, however, the separated peptides stay together in a complex. At the final stage in the secretory granule, the mature oxytocin is no longer complexed to neurophysins.
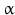 -glycoprotein and
-glycoprotein and 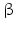 -glycoprotein hormone chains are transcribed from different genes, the oxytocin and the neurophysins are coded for in the same gene and transcribed into a single protein which is then processed during hormone maturation, however, the separated peptides stay together in a complex. At the final stage in the secretory granule, the mature oxytocin is no longer complexed to neurophysins.
-glycoprotein hormone chains are transcribed from different genes, the oxytocin and the neurophysins are coded for in the same gene and transcribed into a single protein which is then processed during hormone maturation, however, the separated peptides stay together in a complex. At the final stage in the secretory granule, the mature oxytocin is no longer complexed to neurophysins.4.2.4 Glycosylation
This step again primarily concerns the glycoprotein hormones. Several asparagine residues are substituted with oligosaccharides. In the Golgi apparatus these contain mannose-rich oligosaccharides. These mannoses form a sorting signal that leads the way to the secretory granules. In later vesicles the mannoses are partially replaced with other sugars and acquire fucoses and terminal N-acetylneuraminic acids , the latter the characteristics of mature glycoproteins. Addition of sugars and replacement of mannoses are common processes of glycoprotein synthesis and not restricted to hormones.
4.2.5 Prohormone Convertases
4.2.5.1 Introduction
We now discuss the special pathways of hormone maturation. As shown above, the first processing of the newly formed polypeptide chain is performed by the signal peptidase which removes the signal peptide. Whenever a signal peptide reaches the interior of the ER it is quickly and reliably removed but the chain itself is not yet finished.
By cleaving off the signal peptide, one end of many protein and peptide hormones is exposed. This end is called the amino terminus or N-terminal end. Here we find the name giving 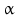 -amino group at carbon atom 1 of the terminal amino acid. Because all the other
-amino group at carbon atom 1 of the terminal amino acid. Because all the other 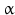 -amino groups are involved in the peptide bonds (boxes in Fig. 4.2), there is only this single
-amino groups are involved in the peptide bonds (boxes in Fig. 4.2), there is only this single 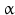 -amino residue in any polypeptide chain. The opposite end of the polypeptide chain is called the carboxy-terminal or C-terminus due to the free carboxy group there, a characteristic feature of organic acids. There is only one free C-terminal carboxy group in any polypeptide inasmuch as all the others are also part of the peptide bonds.
-amino residue in any polypeptide chain. The opposite end of the polypeptide chain is called the carboxy-terminal or C-terminus due to the free carboxy group there, a characteristic feature of organic acids. There is only one free C-terminal carboxy group in any polypeptide inasmuch as all the others are also part of the peptide bonds.
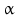 -amino group at carbon atom 1 of the terminal amino acid. Because all the other
-amino group at carbon atom 1 of the terminal amino acid. Because all the other 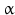 -amino groups are involved in the peptide bonds (boxes in Fig. 4.2), there is only this single
-amino groups are involved in the peptide bonds (boxes in Fig. 4.2), there is only this single 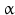 -amino residue in any polypeptide chain. The opposite end of the polypeptide chain is called the carboxy-terminal or C-terminus due to the free carboxy group there, a characteristic feature of organic acids. There is only one free C-terminal carboxy group in any polypeptide inasmuch as all the others are also part of the peptide bonds.
-amino residue in any polypeptide chain. The opposite end of the polypeptide chain is called the carboxy-terminal or C-terminus due to the free carboxy group there, a characteristic feature of organic acids. There is only one free C-terminal carboxy group in any polypeptide inasmuch as all the others are also part of the peptide bonds.
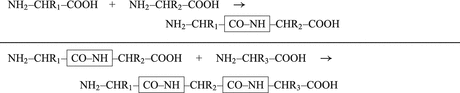
Fig. 4.2
Forming peptide bonds. R1, R2, and R3 represent different amino acid side-chains (Table 16.2)—for example, R1 is CH3; this amino acid is called alanine; two alanines (R1 and R2 are CH3) give rise to alanylalanine; and by adding a third alanine (R3 is CH3), we obtain alanylalanylalanine (Ala–Ala–Ala or AAA)
The C-terminus of almost any vertebrate protein/peptide hormone is exposed by enzymes that recognize dipeptide motifs formed by lysine (K) and arginine (R) and cleave the polypeptide chain behind these dipeptides. These enzymes were labeled prohormone convertases (PC) because they convert the precursor chains into functional hormones (at least sometimes).
4.2.5.2 Sequences and Genes
PC1
The human PC1 gene (other names: neuroendocrine convertase 1 (NEC1); prohormone convertase 3 (PC3)) is found on chromosome 5 at locus 5q15–21 and holds 14 exons. Its promoter is preferentially stimulated by cAMP, and also by, for example, CRH. This suggests coordinated activation of the hormone precursor proopiomelanocortin (POMC) and of its processing enzyme PC1.
The protein PC1 is a serine protease of the subtilisin/kexin type.1
PC2
Twelve exons of the human PC2 gene are distributed on chromosome 20 (20p11.2). The protein precursor is formed in an inactive form and requires for its activation the coexpression of the protein 7B2 (SGNE1). Defects in either of these two genes result in hypoglycemia, hyperinsulinemia, and hypoglucagonemia, indicating that these enzymes participate in insulin precursor processing. The pathological effects are more pronounced when 7B2 is defective compared with PC2 defects.
4.2.5.3 Properties and Physiology
Prohormone convertases cleave an inner peptide bond of polypeptides. Thus, they belong to the large group of endopeptidases. Some endopeptidases cleave the bond between any two amino acids, for example, proteinase K. Others such as trypsin or chymotrypsin recognize single amino acids and cleave the polypeptide chain after these monoamino acid motifs. Prohormone convertases 1 and 2, however, recognize diamino acid motifs with lysine (K) and arginine (K).2 The dibasic amino acid motifs are KK , KR , RK and RR .
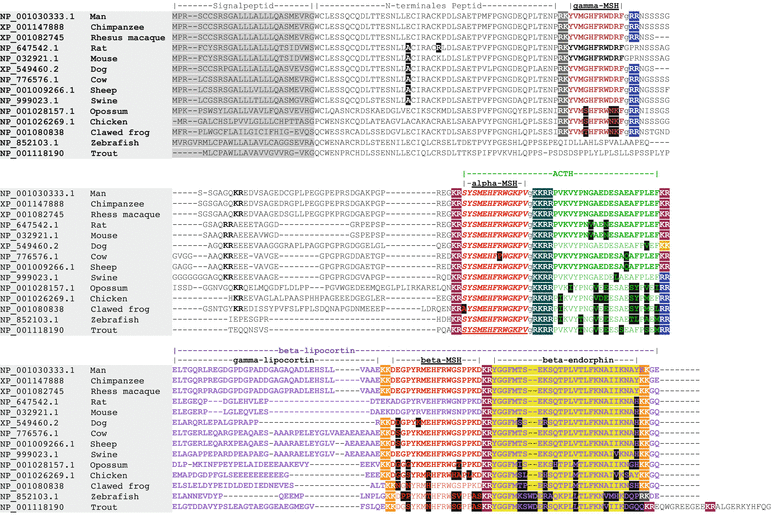
While PC1 preferentially cleaves behind the motif KR ( = lysyl-arginyl)3 all four motifs are recognized and cleaved by PC2. During processing of the POMC precursor this is most important. POMC gives rise to different peptides depending on the PC active in a cell. In addition to adrenocorticotropic hormone (ACTH ) 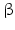 -lipotropin (
-lipotropin (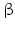 -LPH ),
-LPH ), 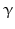 -LPH, β-endorphin , and three distinct melanocyte-stimulating hormones (MSH ) are formed by alternative splicing. A cell with only PC1 derives only ACTH and β-LPH from POMC-like corticotropic cells of the pituitary. Other cells in the brain express PC2 . These cells form
-LPH, β-endorphin , and three distinct melanocyte-stimulating hormones (MSH ) are formed by alternative splicing. A cell with only PC1 derives only ACTH and β-LPH from POMC-like corticotropic cells of the pituitary. Other cells in the brain express PC2 . These cells form 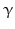 -LPH, β-endorphin and MSHs (Figs. 4.17 and 4.19).
-LPH, β-endorphin and MSHs (Figs. 4.17 and 4.19).
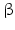 -lipotropin (
-lipotropin (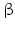 -LPH ),
-LPH ), 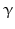 -LPH, β-endorphin , and three distinct melanocyte-stimulating hormones (MSH ) are formed by alternative splicing. A cell with only PC1 derives only ACTH and β-LPH from POMC-like corticotropic cells of the pituitary. Other cells in the brain express PC2 . These cells form
-LPH, β-endorphin , and three distinct melanocyte-stimulating hormones (MSH ) are formed by alternative splicing. A cell with only PC1 derives only ACTH and β-LPH from POMC-like corticotropic cells of the pituitary. Other cells in the brain express PC2 . These cells form 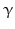 -LPH, β-endorphin and MSHs (Figs. 4.17 and 4.19).
-LPH, β-endorphin and MSHs (Figs. 4.17 and 4.19).The POMC example shows that PC1 or PC2 may cut a precursor chain several-fold. The neuropeptide TRH, for example, from the hypothalamus which induces TSH release in the pituitary exists in six copies in the TRH precursor. Each copy of the peptide sequence QHPG is preceded by a KR motif and followed by a KR or RR motif (Fig. 4.3). PC1 and PC2 produce six oligopeptides from the TRH precursor. TRH is essential for metabolic regulation. Multiplication of its sequence ensures that a single point mutation induces only a gradual loss thus protecting against a dominant TRH defect. Production of multiple copies of a peptide from the same precursor is economical and reduces the energy required for formation of ribosomal complexes and translational start because they are only to be complexed once.
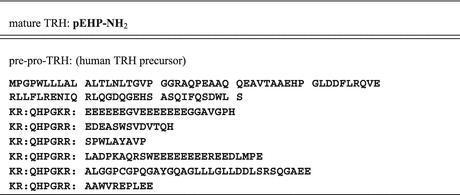

Fig. 4.3
Thyrotropin-releasing–hormone (TRH). The precursor sequence starts at MPG in the upper line, and ends in line 8. The motifs KR and RR are emphasized by colons which also indicates cleavage sites where Prohormone convertase 1 or Prohormone convertase 2 process the precursor. Amino acids are depicted by letters (See Table 16.2)
4.2.5.4 Phylogeny
The mechanisms of hormone formation have not changed much from the very first days of primordial neuropeptides. Thus prohormone convertases are among the primordial enzymes of hormone formation, already found in invertebrates.
4.2.6 Monobasic and Dibasic Sequence Motifsin Invertebrates and Vertebrates
Viewing the many neuropeptide precursors of vertebrate and invertebrate species the common KR sequences are striking. These constitute by far the most frequent peptide motif recognized by prohormone convertase. KR is the PC1 motif. Much less frequent are the other three dibasic motifs KK, RK, or RR recognized by PC2. Sometimes (more in invertebrates, less in vertebrates) we find motifs for furin-like peptidases RxxxxR with two to four variable ( = x) amino acids. Very rarely there are monobasic K or R cleavage sites where in mammals trypsin or chymotrypsin would cleave the chain.
Veenstra (2000) and Southey et al. (2008, 2006) have reviewed that KR sites are always used whereasRR, KK, or RK sites are less frequently used. The utilization of monobasic recognition sites is not yet understood because the identity and even more the specificity of enzymes in different taxonomical orders are far from being fully understood. Sometimes furin-like and dibasic sites in the same precursor are used: for example, the short neuropeptide F (sNPF) precursor from the mosquito Anopheles gambiae (Fig. 5.31) is cleaved into five, from the same extract chemically identified oligopeptides. Three of these are cleaved after a dibasic, however, two of them in a furin-like motif.
4.2.7 Chopping the C-Terminus
By cleaving the TRH precursor chain at the KR motif, the maturation process is not yet finished. In many cases this peptide is still nonfunctional. Comparing the different TRH-cleavage products with mature TRH, we observe that they are still extended at the C-terminus.
Other peptidases than those described thus far will now chop off all amino acids from the C-terminus until they encounter a glycine residue. Glycine cannot be removed by these enzymes. Thus QHPGKR will be left from QHPG, however, QHPG will also remain from QHPGRR:EDEASWSVDVTQH because there is no glycine but that in position 4; all the other amino acids will be sequentially removed from the C-terminus. After similar processing of the other four oligopeptides six QHPG peptides are present.
4.2.8 Oxidation of the Terminal Glycine
The peptidyl glycine- -amidating monooxygenase (PAM ) oxidizes the terminal glycine into an amide residue (Fig. 4.4). At first the
-amidating monooxygenase (PAM ) oxidizes the terminal glycine into an amide residue (Fig. 4.4). At first the  -C atom of glycine will be oxidized. This reaction is only possible in glycine with its two hydrogen atoms at the C
-C atom of glycine will be oxidized. This reaction is only possible in glycine with its two hydrogen atoms at the C atom. The second step involves removal of glyoxal and leaves the NH2 function. Because this is coupled to a carbonyl double bond the structural name is amide. Amides are less prone to chemical attack than amino groups. Amidation of the C-terminus increases the overall survival of a peptide in the body where many enzymes are ready to digest a lonely peptide.
atom. The second step involves removal of glyoxal and leaves the NH2 function. Because this is coupled to a carbonyl double bond the structural name is amide. Amides are less prone to chemical attack than amino groups. Amidation of the C-terminus increases the overall survival of a peptide in the body where many enzymes are ready to digest a lonely peptide.
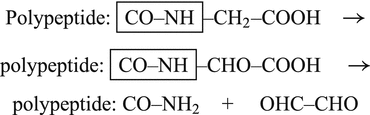
 -amidating monooxygenase (PAM ) oxidizes the terminal glycine into an amide residue (Fig. 4.4). At first the
-amidating monooxygenase (PAM ) oxidizes the terminal glycine into an amide residue (Fig. 4.4). At first the  -C atom of glycine will be oxidized. This reaction is only possible in glycine with its two hydrogen atoms at the C
-C atom of glycine will be oxidized. This reaction is only possible in glycine with its two hydrogen atoms at the C atom. The second step involves removal of glyoxal and leaves the NH2 function. Because this is coupled to a carbonyl double bond the structural name is amide. Amides are less prone to chemical attack than amino groups. Amidation of the C-terminus increases the overall survival of a peptide in the body where many enzymes are ready to digest a lonely peptide.
atom. The second step involves removal of glyoxal and leaves the NH2 function. Because this is coupled to a carbonyl double bond the structural name is amide. Amides are less prone to chemical attack than amino groups. Amidation of the C-terminus increases the overall survival of a peptide in the body where many enzymes are ready to digest a lonely peptide.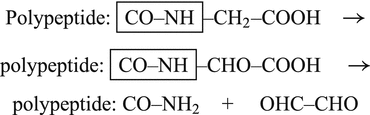
Fig. 4.4
Amidation of the C-terminal glycine
Remember that precursor sequences such as peptide-GxxKR peptide-GxxRR, peptide-GxxRK, or peptide-GxxKK will result in a peptide-amide at the C-terminal end of the hormone (xx indicates small or larger peptide sequences and may also be missing).
4.2.9 Cyclization of the N-Terminal Glutamine
The perseverance of a peptide hormone in the circulation will be further enhanced by one additional step of hormone maturation: the N-terminal glutamine (Q) will undergo intramolecular cyclization giving rise to a N-terminal pyroglutamic acid group (pE; Fig. 4.5).
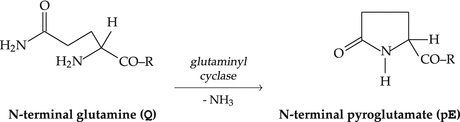

Fig. 4.5
Cyclization of the N-terminal glutamine
The neuropeptide gonadotropin-releasing hormone thus loses its last free amino group. Such a peptide, especially if no lysyl residue is present that has an additional ε-amino group, is better armed against enzymatic degradation. Given that GnRH need only survive for a little more than 2 cm of bloodstream its half-life of 5 min in blood is sufficiently long enough to ensure that the receptors on the pituitary cells get triggered .
4.2.10 Esterification of Ghrelin
Without precedent among secreted peptides is esterification of ghrelin by octanoic acid. O-Acyltransferase—that is, the enzyme that transfers octanoic acid to the hydroxyl group of serine at position 3 of ghrelin—has been identified (Yang et al. 2008; Gutierrez et al. 2008).
Other peptides with long-chain fatty acid substitutions have these at their N-terminus or at the free lysyl amino groups. The reversible transfer of palmitic acid to the cysteine of guanosine nucleotide-binding proteins (G proteins) while forming a thioester bond suggests a special function for this modification. The acylated G-protein complexes associate with membranes and may thus facilitate hormone receptor interactions (see also Sect. 8.2.1). Gene activation by acetylation of histones also belongs to these mechanisms.  -Endorphins and
-Endorphins and  -melanocortins are also N-terminally acetylated.
-melanocortins are also N-terminally acetylated.
 -Endorphins and
-Endorphins and  -melanocortins are also N-terminally acetylated.
-melanocortins are also N-terminally acetylated.Apart from octanoic acid other fatty acids including decanoic acid and its unsaturated decenoic acid have also been found as substituents of ghrelin. We would assume that further chain elongation might result in strong unspecific interactions of ghrelin with any membranes. A hormone with such long-chain fatty acids will never reach its receptor because it previously got stuck somewhere.
4.3 Peptide Hormones of the Hypothalamus and the Brain
4.3.1 Hypothalamic-Releasing Hormones
GnRH, TRH, CRH, GHRH (Table 4.1): These four neuropeptides stimulate release of hormones in the pituitary: GnRH induces the release of the gonadotropins, LH and FSH , TRH of thyrotropin (thyroid-stimulating hormones, TSH), CRH boosts corticotropin (ACTH ; adrenocorticotropic hormone) release, and GHRH stimulates growth hormone (GH ; older term somatotropin) secretion. After being formed in neurosecretory cells of the hypothalamus (see Sect. 10.2.1), the four neuropeptides are transferred via axonal transport into the median eminence where they will be released by appropriate stimuli. The blood capillaries will be reached by diffusion through little windows in the capillary wall. By direct transport through a portal system the four releasing hormones reach the anterior pituitary and their cellular targets leaving the capillaries again in fenestrated areas .
Such fenestrated passages between brain cells and blood vessels are called neurohemal organ s. Usually the vessels in the brain are covered with a thickened layer of cells, the blood–brain barrier (BBB); in neurohemal organs the BBB is missing and a direct transport of hormones into and from the blood is permitted.
Table 4.1
The hypothalamic-releasing hormones (RH)

Released in the median eminence the four neuropeptides reach the pituitary straight via a portal system. The distance is not much larger than 2 cm. During this short passage the peptides are stable. In the pituitary there are again fenestrated capillaries allowing the hormones to reach the receptors on the target cells.
4.3.1.1 TRH
Fact sheet 4.1: Thyrotropin-Releasing Hormone
Gene:
Chromosome 3; locus 3q13.3-q21; three exons.
Sequence:
pEHP-NH 2.
Synthesis and target:
TRH is preferentially synthesized in the paraventricular nucleus and acts via the median eminence on thyrotropic and lactotropic cells of the pituitary. TRH is also active as a neurotransmitter in many neurons.
Function:
Releasing hormone for thyrotropin and prolactin; major stimulator of metabolism; controls thyroid gland functions; equally active as neurotransmitter.
Receptor:
Heptahelical GPC membrane receptor.
Introduction
TRH was the first hypothalamic neuropeptide whose structure could be determined in 1969 (Boler et al. 1969; Burgus et al. 1969). About 500 tons of sheep brain were used to extract the peptide and identify the structure pyrGlu-His-Pro-NH2. Compared to usual peptides TRH shows three distinct characteristics:
1.
It is very short, only a tripeptide.
2.
The C-terminus is amidated.
3.
The N-terminus is a pyroglutamic acid.
Inasmuch as TRH was the first neuropeptide whose structure was determined, these features were very new; TRH appears to be the proverbial needle in the haystack to be looked for. The problems the protagonists in the race for the first neuropeptide structure, Schally and Guillemin, encountered can be studied in the book by Crapo (1985)
Biochemistry and Genes
On chromosome 3 (3q13.3-a21) the singular gene for the TRH precursor was found to contain three exons. After splicing and translation, the precursor contains several copies of the QHPG sequence; the KR prohormone convertase 1 recognition site is present several-fold, too. By PC1 the precursor is cleaved and the several precursor peptides then undergo maturation to the final TRH: pEHP-NH2 (see Sect. 4.2). There are six copies of the QHPG sequence in the TRH precursor in humans, five in rats, and seven in frogs.
Physiology
TRH is the major regulator of the thyroid hormone and thus of energy homeostasis. In “lower” vertebrates TRH functions as a neurotransmitter because these animals do not synthesize thyrotropin. This neurotransmitter function is also retained in mammals, independently of the hypothalamic–pituitary–thyroidal axis.
Apart from the hypothalamus pro-TRH is synthesized in many brain regions: in the reticular nucleus of the thalamus , in the cerebral cortex , in pyramidal cells of the hippocampus , in external “plexiformal” layers of the olfactory bulb , in the sexually dimorphic nucleus , in the preoptic area, in the supraoptic nucleus , and in the substantia nigra , as well as in the pineal gland and the spinal cord.
Nonneural tissues where TRH is expressed are the mammalian pancreas and normal thyroid tissue. Frogs express TRH in their skin.
Human TRH regulates a circadian TSH rhythm with maximal release at midnight and minimal concentrations in the late afternoon. There are additional ultradian TSH peaks in 2- to 4-h intervals (see also Chap. 12). These rhythms are controlled by the suprachiasmatic nucleus and other cerebral pacemakers (Chap. 12). The limbic system , the pineal gland , and CNS regions involved in stress responses (Sect. 11.2.1) co-influence the pulsatile TRH/TSH release.
Catecholamines are further important regulators of hypothalamic TRH neurons: 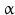 1-adrenergic neurons from the brainstem activate hypothalamic TRH neurons. Noradrenaline induces in vitro TRH secretion and dopamine inhibits TSH release. Application of the tyrosine hydroxylase inhibitor
1-adrenergic neurons from the brainstem activate hypothalamic TRH neurons. Noradrenaline induces in vitro TRH secretion and dopamine inhibits TSH release. Application of the tyrosine hydroxylase inhibitor 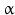 -methyl-p-tyrosine diminishes the TSH release triggered by chilling (compare catecholamine biosynthesis, Fig. 7.1).
-methyl-p-tyrosine diminishes the TSH release triggered by chilling (compare catecholamine biosynthesis, Fig. 7.1).
 1-adrenergic neurons from the brainstem activate hypothalamic TRH neurons. Noradrenaline induces in vitro TRH secretion and dopamine inhibits TSH release. Application of the tyrosine hydroxylase inhibitor
1-adrenergic neurons from the brainstem activate hypothalamic TRH neurons. Noradrenaline induces in vitro TRH secretion and dopamine inhibits TSH release. Application of the tyrosine hydroxylase inhibitor 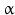 -methyl-p-tyrosine diminishes the TSH release triggered by chilling (compare catecholamine biosynthesis, Fig. 7.1).
-methyl-p-tyrosine diminishes the TSH release triggered by chilling (compare catecholamine biosynthesis, Fig. 7.1).Endogenous opioids as well as somatostatin block TRH release; the latter blocks TSH release as well.
By glucocorticoid s TRH mRNA transcription is directly blocked and by stimulating somotastatin indirectly. Dexamethason, a synthetic glucocorticoid, however, stimulates TRH transcription. In vivo such directly stimulating effects are counteracted by inhibitory neural influences from, for example, the hippocampus.
In its role as neurotransmitter TRH is involved in thermoregulation and in the amplification of noradrenergic and dopaminergic effects. By stimulating the preoptic area a direct influence on the regulation of body temperature is exerted. While activating the thyroid gland and thus metabolic activity, TRH indirectly enhances the body temperature and the activity of sympathetic neurons in the brainstem and the spinal cord.
Phylogeny
TRH is a characteristic vertebrate hormone. In agnathans (hagfish and lampreys) TRH positivity has been observed by immunocytology. Related peptides have been observed in lancelets and echinoderms: pESP-amide in lancelets and pEWP-amide and pEYP together in a common precursor protein. Multiple copies of the sequences are found as in the human TRH precursor. Teleosts, frogs, birds, and mammals all express one homologous gene in the brain with the translated sequence QHPG. Maturation to the active pEHP-NH2 is found in all these vertebrates. In Xenopus laevis, a second gene was identified that also shows seven peptide copies. This gene, however, has a different promoter and is predominantly expressed in the frog’s skin. At least one of the TRH receptors is expressed in the Xenopus laevis skin, which suggests that color adaption to the environment might be regulated by TRH.
4.3.1.2 CRH
Fact sheet 4.2: Corticotropin-releasing hormone
Gene:
Chromosome 8; locus 8q13; two exons
Sequence:
SEEPPISLDL TFHLLREVLE MARAEQLAQQ
AHSNRKLMEI I-NH2.
Synthesis and target:
CRH is predominantly synthesized in the paraventricular nucleus, released in the median eminence and targets corticotropic cells of the pituitary; CRH is released from the placenta.
Function:
Releasing hormone for ACTH; central regulator of neuroendocrine reactions and behavior in response to stress; during gestation a potential indicator of preterm delivery.
Receptor:
Two heptahelical GPC membrane receptors, CRHR1 and CRHR2, with alternatively spliced products.
Introduction
An adequate response to stress in mammals depends on a functional hypothalamic–pituitary–adrenal axis (HPA). CRH, its receptors on corticotropic cells in the pituitary, ACTH released by these cells and its receptors, together with cortisol synthesis and release in the adrenal constitute this HPA. The indispensable role of CRH was demonstrated in the analysis of children suffering from congenital isolated adrenocorticotropic hormone deficiency where an abnormal CRH gene structure or expression was observed.
Biochemistry and Genes
CRH is derived from a preprohormone in a classical way processed as shown in the earlier chapters. The amidated C-terminus is a prerequisite for CRH receptor binding whereas the N-terminus is not required. Thus N-terminally shortened CRH peptides such as the CRH-9–41 peptide are fully biologically active. Oxidation of the methionine residue in position 38 destroys any biological activity which is a way as to inactivate CRH. The human CRH gene is found on chromosome 8 (locus 8q13) (Kellogg et al. 1989).
Physiology
CRH and vasopressin are the primary hormonal regulators of the human stress response. The observation of CRH and its receptors in the brain region apart from the hypothalamus, for example, as in the limbic system, in the central, stimulating sympathetic system of the brainstem and the spinal cord suggest this role. Intracerebral injection of CRH in animals leads to a coordinated sequence of physiological and behavioral reactions. These comprise:
Activation of the hypothalamic–pituitary–adrenal axis
Activation of the system of the nervus sympathicus
Enhanced alertness
Suppression of feeding and sexual activity
Hypothalamic hypogonadism
Changes in locomotor activity
These items characterize the usual behavior when stressed.
There are additional allies of this response which function as important regulators of corticotropic cells. A mutual positive interaction exists between CRH and vasopressin (AVP ) release in the hypothalamopituitary unit: AVP induces CRH release and CRH stimulates AVP release. Without stress the pulses of these two hormones are more than 80 % overlapping. During stress the amplitude enlarges and if magnocellular AVP neurons are involved a continuous increase of the AVP level in plasma is observed.
CRH as well as AVP are released after stimulation by catecholamines (dopamine , noradrenaline , and adrenaline ). AVP/CRH neurons on the one hand and the locus coeruleus plus noradrenergic neuron s of the central stress response system are intimately mutually innervated and are regulated by the same factors in parallel. There are some ultrashort feedback loops by CRH on CRH neurons and by noradrenalin on noradrenergic neurons (Strakis and Chrousos 1997).
CRH and noradrenergic neurons as well are triggered by serotonin (5-HT) and acetylcholine and inhibited by corticosteroids and by the neurotransmitter γ-aminobutyric acid (GABA ). The peptides derived from POMC and released after CRH stimulation in the pituitary such as ACTH , α-MSH , 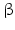 -endorphin and further opioids such as dynorphin , exert a feedback inhibition on CRH and on noradrenergic neurons. Intracerebral injection of noradrenaline upregulates CRH, AVP, and ACTH release in the CNS, but not the ACTH secretion in the pituitary. Catecholamines, therefore, influence brain regions that are upstream of the pituitary functions and thus enhance AVP and CRH release.
-endorphin and further opioids such as dynorphin , exert a feedback inhibition on CRH and on noradrenergic neurons. Intracerebral injection of noradrenaline upregulates CRH, AVP, and ACTH release in the CNS, but not the ACTH secretion in the pituitary. Catecholamines, therefore, influence brain regions that are upstream of the pituitary functions and thus enhance AVP and CRH release.
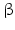 -endorphin and further opioids such as dynorphin , exert a feedback inhibition on CRH and on noradrenergic neurons. Intracerebral injection of noradrenaline upregulates CRH, AVP, and ACTH release in the CNS, but not the ACTH secretion in the pituitary. Catecholamines, therefore, influence brain regions that are upstream of the pituitary functions and thus enhance AVP and CRH release.
-endorphin and further opioids such as dynorphin , exert a feedback inhibition on CRH and on noradrenergic neurons. Intracerebral injection of noradrenaline upregulates CRH, AVP, and ACTH release in the CNS, but not the ACTH secretion in the pituitary. Catecholamines, therefore, influence brain regions that are upstream of the pituitary functions and thus enhance AVP and CRH release.AVP and CRH neurons additionally release products of the dynorphin gene together with AVP or CRH. These products including 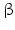 -endorphin are potent endogenous opioids and suppress AVP and CRH effects on target cells.
-endorphin are potent endogenous opioids and suppress AVP and CRH effects on target cells.
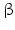 -endorphin are potent endogenous opioids and suppress AVP and CRH effects on target cells.
-endorphin are potent endogenous opioids and suppress AVP and CRH effects on target cells. CRH-Binding Protein
In addition to the CRH receptor there is a plasma CRH-binding protein (CRH-BP) with high affinity for CRH. Binding to this binding protein blocks activation of the CRH receptor by CRH. The binding protein is not related to the receptor. It has been found in the CNS, in placenta, in the amnion liquid, and in human plasma. In mice and cows, however, the binding protein has not been identified in plasma. Expression of the CRH-BP in the brain modulates reactions to stress. Deletion of the CRH-BP gene in mice increases anxiety in these mice but not in control animals. As mice do not have CRH-BP in their plasma or in the adrenal glands, there is no effect of the deletion in modified mice (Karolyi et al. 1999).
Phylogeny
Vertebrates possess three further CRH-related genes involved in the response to stress: CRH/CRF, urocortin/urotensin I, stresscopin (SCP)/urotensin III, and stresscopin-related peptide (SRP)/urotensin II. Between mammals and teleosts sequence homology is above 96 % for CRH/CRF, and above 55 % for SCP. There are similar precursor proteins and derived peptides in insects. This suggests that fight-or-flight responses and the handling of stress might have been present early in chordate evolution and that the two CRH receptors have mediated these responses.
Human urocortin (40 amino acids long and coded for on chromosome 2) preferentially binds to CRH receptor 2. Its effect is diminishing appetite and not to mediate stress. Stresscopin (40 amino acids) and stresscopin-related peptide (coded for on chromosome 3 (3q21.3; 43 amino acids)) display similar reactions. This might indicate that CRH mediates immediate reaction to stress by inducing cortisol synthesis and release and managing the metabolic changes depends on CRH, but equally on urocortin, stresscopin, or SCP.
The homology between vertebrates and insects extends to the CRH binding protein (Huising and Flik 2005). This extends the discussion of whether CRH might have already been present in common ancestors of insects and vertebrates.
4.3.1.3 GnRH
Fact sheet 4.3: Gonadotropin-Releasing Hormone
Genes:
GnRH-I: Chromosome 8; locus 8p21-p11.2; four exons
GnRH-II: Chromosome 20; locus: 20p13; four exons
Sequences:
GnRH I: pEHWSY GLRPG-NH2
GnRH II: pEHWSH GWYPG-NH2.
Synthesis and target:
GnRH-I is expressed in several nuclei of the hypothalamus, released in the median eminence and triggers pituitary gonadotropic cells. Additionally, GnRH is expressed in trophoblastic cells of the placenta, to a lesser degree by T-lymphocytes.
GnRH-II is preferentially expressed in kidney, bone marrow, and prostate; in the brain in the caudate nucleus, the hippocampus, and the amygdala.
Function:
GnRH-I: releasing hormone of LH and FSH; central regulator of reproduction; during gestation stimulator of choriogonadotropin
. GnRH-II: found to enhance ovarian cancer invasiveness.
Receptor:
One GPCR: GnRHR1 (the human GnRHR2 gene is a pseudogene).
Introduction
GnRH-I is the major regulator of vertebrate reproduction. Its sequence is identical for almost all mammalian species (with the known exception of guinea pigs). Other vertebrates possess this mammalian GnRH-I such as certain teleost fish or frogs. Another peptide, GnRH-II, very similar to GnRH I, is labeled according the species where it was identified: chicken-II. In fish the first GnRH is seabream GnRH (sbGnRH), the second GnRH-II, also known as chII-GnRH, and a third GnRH-III, also known as salmon-GnRH (smGnRH). Although GnRH-II is predominantly expressed in the forebrain of fish, the other two GnRH are found in midbrain. sbGnRH is the hypothalamic hormone.
Biochemistry and Genes
The human genes on chromosomes 8 and 20 are similarly organized, the introns being larger in the GnRH-I gene on chromosome 8. Alternative splicing of the GnRH-II mRNA enlarges the polypeptide by several amino acids; this alternative splicing appears tissue specific (White et al. 1998).
Using the GnRH precursor the decapeptide GnRH is formed by the sequential action of the following enzymes:
1.
the signal peptidase
2.
the prohormone convertase PC1
3.
the exopeptidase E
4.
the peptidylglycyl 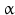 -amidating monooxygenase (PAM )
-amidating monooxygenase (PAM )
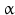 -amidating monooxygenase (PAM )
-amidating monooxygenase (PAM )5.
the glutaminyl cyclase
A large collection of synthetic structural analogues was instrumental in identifying structure–function relationships:
N- and C-terminus are required for receptor binding.
Amino acids (AA) 1–4 are necessary to release LH or FSH.
The side-chains of His2–yr5–Arg8 are essential for full biological activity.
Replacement of Arg8 decreases LH and FSH secretion.
Changing Gly6 for Leu6 influences the capacity for LH secretion in a more profound way than the activity to release FSH.4
The secondary structure of all GnRH peptides is conserved , because the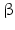 -turn, formed by amino acids 5–8, induces a hairpin loop required for receptor binding.
-turn, formed by amino acids 5–8, induces a hairpin loop required for receptor binding.
Physiology
Mammalian reproduction depends on secretion of hypothalamic GnRH in all species analyzed thus far. Its regulation is controlled by multiple set points, hormones, neurotransmitters, and regulator circuits (see also Sect. 11.3).
Most critical for functional GnRH activity is pulsatile release of GnRH. Without this periodic and (only in adults) fully adjusted release pattern, any LH and FSH secretion does not take place; on the contrary continuously elevated GnRH levels in the blood lead to LH and FSH suppression. This constitutes one mechanism of contraception (see also Sect. 11.3).
During fetal development, GnRH-positive neurons migrate from the olfactory bulb to the hypothalamus. Any disturbance of this migration results due to missing hypothalamic GnRH neurons in infertility frequently combined with olfactory defects (Kallmann syndrome).
The physiology of GnRH-II5 is far from being understood. Due to its conserved structure for 500 million years a critical role is suggested. On the other hand, in cow and sheep, the gene is present as well as the gene for the GnRH2 receptor. However, there is a mutation in the cow sequence that prohibits receptor binding and the sheep gene harbors a premature stop codon that abolishes any GnRH-II synthesis. The human GnRH2 receptor is afunctional owing to a frameshift mutation. GnRH-II binds the GnRH1 receptor, however, with a signal induction different from GnRH-I induction. An important functional role is thus not evident at all.
The teleost GnRH-III is reported to be expressed in the forebrain whereas GnRH-I and GnRH-II have been found in the diencephalon. The GnRH-III neurosecretory cells are located close to the nervus terminalis, not far from the olfactory bulb. Their axons reach the retina. It has been suggested that GnRH-III may control pattern recognition in animals ready to mate. It is worth noting that GnRH-I neurons originally were observed in the very same brain region. These latter, however, migrate to the hypothalamus whereas the GnRH-III neurons stay in the forebrain. In vertebrates other than teleosts, a GnRH-III gene has not been found. There are further GnRH-like genes in agnathans; these are, however, not related to the GnRH-II gene.
Phylogeny
For a long time, it was assumed that GnRH peptides were characteristic vertebrate hormones. This assumption has been discarded. There are three GnRH peptides in lampetra, presumably evolutionarily older than chondrychthyes and osteichthyes or newer vertebrates; molluscs such as Aplysia californica and Octopus vulgaris were shown to form a GnRH-like peptide of 12 amino acids, with the insertion at the same place. Ciona intestinalis expresses nine GnRH peptides and a 16 amino acid long GnRH-like peptide that differs from all other peptides by an elongation at the C-terminus (Table 4.2). Reports in corals about GnRH activity that could release LH from teleost cells have not been corroborated by peptide sequencing or cDNA cloning (Twan et al. 2006).
Table 4.2
Sequences of GnRH variants with mammalian GnRH as a reference
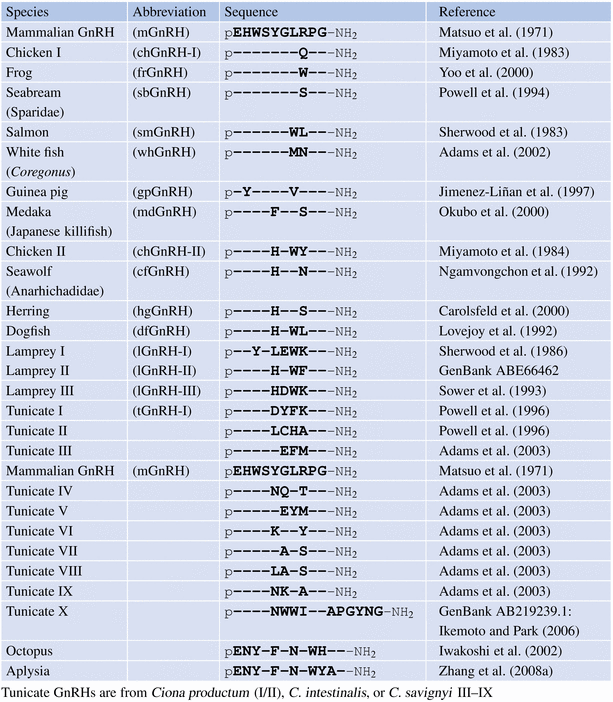
The figure from Guilgur et al. (2006) was used as a template to generate a phylogenetic tree that includes nonvertebrate GnRH sequences.
Figure 4.6 shows the characteristic three GnRH types of fish. Some species have two chII-GnRH precursors, goldfish and carp probably indicating the duplication of the entire genome.

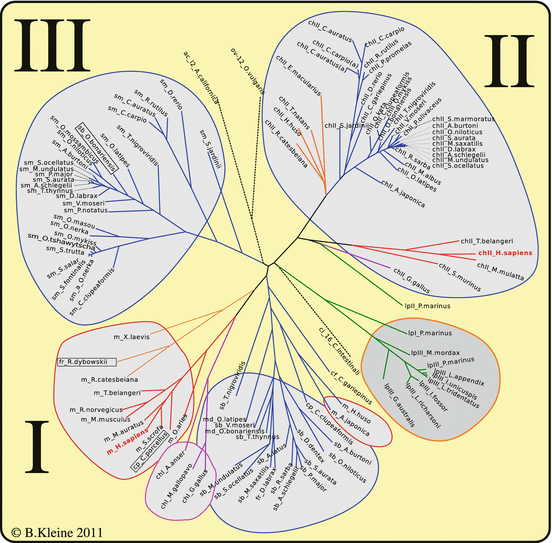


Fig. 4.6
Phylogenetic tree of GnRH variants
The ClustalW algorithm sorts the sequences first of all due to the sequence of the mature peptides (whereas the input comprised the whole precursor proteins). Further differentiation occurs due to differences in the remaining sequences, signal peptide, or associated peptide. There are additional differences separating chII-GnRH of mammals and birds. Although any variations of chII-GnRH have not been observed, smGnRH-II and GnRH (m-, chI- or sb-GnRH) show single amino acid exchanges (boxed in Fig. 4.6).
The pattern gains further complexity if we include the GnRH receptors. Up to five distinct GnRH receptor genes have been found (e.g., in the Takifugu rupripes genome (http://genome.jgi-psf.org/Takru4/Takru4.home.html) and in seabream (Moncaut et al. 2005). Some degree of tissue-specific differential expression of different receptor genes does not allow a definite association of receptor type and GnRH variant with any function. For this reason, the situation in mammals with the hypothalamic GnRH secretion and the derived pituitary gonadotropin release appears functionally clear. We discuss it again in Sect. 11.3.
4.3.1.4 GHRH
Fact sheet 4.4: Growth hormone releasing hormone
Gene:
Chromosome 20; locus 20q11.2; five exons.
Sequence:
YADAIFTNSY RKVLGQLSAR KLLQDIMSRQ QGESNQERGA RARL-NH2.
Synthesis and target
GHRH neurons exist in the ventromedial nucleus and the arcuate nucleus; they secrete in the median eminence .
Function:
Releasing hormone of the pituitary growth hormone.
Receptor:
GPC heptahelical membrane receptor.
Introduction
Release of the growth hormone in the pituitary is regulated by stimulating (GH releasing hormone; GHRH) and inhibiting (somatostatin) neuropeptides. Both are secreted in the median eminence . Recently, ghrelin was identified to stimulate GH release, too.
Structure and Genes
The GHRH gene maps to chromosome 20q11.2. The translated RNA gives rise to a prepropolypeptide that contains a 30 amino acid long signal peptide, the GHRH sequence (1–44), the amidation signal, and the 30 or 31 amino acid long stretch of the C-terminal peptide. GHRH is posttranslationally modified like the other neuropeptides: cleaved by the signal peptidase and then by the prohormone convertase-1, shortened by endopeptidases, and finally amidated by PAM. Endopeptidase treatment at the C-terminal region forms 40 or 37 amino acid long, biologically active peptides. Further digestion to a 29 amino acid long peptide abolishes any biological activity.
Physiology
GHRH release is controlled by product feedback; it is growth hormone regulated . In the majority of brain regions the GH receptor and GHRH RNA were co-localized: in the hypothalamus , thalamus , septal region , hippocampus , dentate gyrus , or amygdala .
GHRH expression is higher in the hypothalami of male rats than in female hypothalami. This sexually dimorphic behavior is controlled by steroid hormones: Dihydrotestosterone (DHT ) injection into ovariectomized rats masculinized their GH secretion. Injection of estradiol, however, diminished the GHRH secretion in male rats. In addition, the GH feedback control of GHRH release appears gender specific.
Those neurosecretory cells that release GHRH in the median eminence are found in the ventromedial nucleus and in the arcuate nucleus of the hypothalamus. They are interconnected with different CNS areas: signals from the “sleep center(s) ” are stimulating and coupled to the sleep rhythm. Signals from the amygdala and from ascending noradrenergic neurons of the brain stem are related to the activation of the stress reaction. These mediate stress-induced GH release. The ventromedial nucleus processes the secretion of hormones involved in blood glucose regulation and thus influences the GHRH release in reaction to hypoglycemia (see also Sect. 11.4).
GH release is regulated by GRHR stimulation and somatostatin (SST) inhibition. Functional and anatomically reciprocal interactions exist between the ventromedial nucleus, arcuate nucleus, and paraventricular nucleus: endogenous SST inhibits GHRH secretion from median eminence , whereas intracerebral SST injection stimulates GHRH release. GHRH neurons of the arcuate nucleus express high-affinity SST receptors. In addition to SST regulation, circadian GHRH pulses are controlled by zeitgeber of the suprachiasmatic nucleus. This circadian GHRH rhythm is synchronized to the sleep rhythm: elevated GH secretion during sleep, reduced GH release while awake.
GHRH neurons are further influenced by other neurons and their neurotransmitters: sleep-induced GH release is modulated by serotonergic and cholinergic neurons . Circadian GH pulses mediated by GHRH may be inhibited by 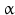 -antagonists i.e. inhibitors of catecholamine α-receptors , or substances directly blocking catecholamine biosynthesis.
-antagonists i.e. inhibitors of catecholamine α-receptors , or substances directly blocking catecholamine biosynthesis. 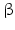 2-Agonist , stimuli of the
2-Agonist , stimuli of the 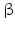 2-catecholamine receptor , induce GH release presumably by stopping SST secretion. Anticholinergic drugs inhibit all GH stimulating effects but hypoglycemia. L-DOPA6 as well as dopamine increase GH release most probably due to their local conversion into noradrenaline.
2-catecholamine receptor , induce GH release presumably by stopping SST secretion. Anticholinergic drugs inhibit all GH stimulating effects but hypoglycemia. L-DOPA6 as well as dopamine increase GH release most probably due to their local conversion into noradrenaline.
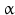 -antagonists i.e. inhibitors of catecholamine α-receptors , or substances directly blocking catecholamine biosynthesis.
-antagonists i.e. inhibitors of catecholamine α-receptors , or substances directly blocking catecholamine biosynthesis. 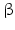 2-Agonist , stimuli of the
2-Agonist , stimuli of the 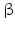 2-catecholamine receptor , induce GH release presumably by stopping SST secretion. Anticholinergic drugs inhibit all GH stimulating effects but hypoglycemia. L-DOPA6 as well as dopamine increase GH release most probably due to their local conversion into noradrenaline.
2-catecholamine receptor , induce GH release presumably by stopping SST secretion. Anticholinergic drugs inhibit all GH stimulating effects but hypoglycemia. L-DOPA6 as well as dopamine increase GH release most probably due to their local conversion into noradrenaline.Apart from SST other CNS neuropeptides interact with GHRH neurons and contribute to GH release:
Endogenous endorphin s, in particular -endorphin, increases GHRH and GH release.
-endorphin, increases GHRH and GH release.
TRH, injected in to rat brain enhances GH release by a Ca2+-dependent, cAMP-independent mechanism. In humans, TRH injection increases GH levels only in patients with acromegaly.
Galanin , motilin and NPY7 stimulated GH secretion from isolated rat pituitary cells. A subgroup of GHRH neurons themselves expresses neuropeptide Y which in vitro appears to upregulate GH release . Applied into a cerebral ventricle NPY quenches GH secretion suggesting additional regulators of GHRH and SST neurons by the inhibiting ascending noradrenergic neurons from the brainstem usually stimulating GH secretion via GHRH.
Phylogeny
Phylogenetically GHRH is closely related to another neuropeptide, PACAP.8 By gene duplication at the beginning of mammalian evolution two different genes for GHRH and PACAP were formed. Nonmammalian vertebrates form GHRH and PACAP by alternatively splicing the same RNA precursor. It is worth noting that the PACAP sequence is more strongly conserved in mammals than is the GHRH sequence (Montero et al. 2000).
After the event of gene duplication the GHRH exon in the PACAP gene gave rise to the PACAP-related peptide (PRP) whereas the PACAP exon of the GHRH gene mutated to the C-peptide of GHRH. PACAP is, like GHRH, amidated.
PACAP has been reported as an additional inducer of noradrenaline secretion in the adrenal glands and as a mediator of the metabolic response to elevated blood glucose levels. PACAP knockout mice and flies with PACAP defects show behavioral disorders, in PACAP mice the metabolite 5-hydroxyindoleacetate was reduced.
mice the metabolite 5-hydroxyindoleacetate was reduced.
 mice the metabolite 5-hydroxyindoleacetate was reduced.
mice the metabolite 5-hydroxyindoleacetate was reduced.PACAP and GHRH belong to the family of secretin-like peptides.
4.3.2 Gonadotropin-Inhibiting Hormone
Fact sheet 4.5: Neuropeptide VF; RF-related peptide; aka gonadotropin-inhibiting hormone (GnIH)
Gene:
Chromosome 7; locus 7p15; three exons.
Structure:
See Fig. 4.7; three peptides with a similar C-terminus, LPLRFamide, LPQRFamide, and LPLRSamide.
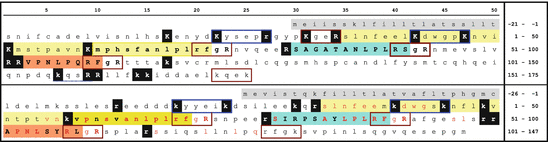

Fig. 4.7
Chicken gonatotropin inhibiting hormone (GnIH) and the human homologue, RFamide-related peptide (RFRP). The upper sequence displays the human preproprotein, the lower that of chicken. Signal peptides are on gray background, RFRP-1 on yellow, RFRP-2 on blue, and RFRP-3 on orange background. Monobasic and dibasic peptid motifs. are inverted. Red and blue frames indicated unusual PC1 motifs. Conserved amino acids are shown red in the chicken sequence. RFRP-1 is labeled by Tsutsui as a presumable LPLRFamide peptide due to the unknown cleavage mechanisms for the N-terminus (Tsutsui 2009) (Source: Swiss-Prot Q9HCQ7 (human) and GenBank BAE17049 (chicken))
Synthesis and target:
Neuropeptide VF is expressed in dorsomedial neurons and in gonads and acts as a neurotransmitter preferentially on GnRH neurons in the median eminence .
Function:
Inhibitor of GnRH release and of gonadal activity.
Receptor:
Neuropeptide FF receptor 1; GPCR147 from the rhodopsin receptor family.
4.3.2.1 Introduction
Any preproproteins of human peptide hormones cleaved to release peptide hormones possess basic dipeptide motifs where either PC1 or PC2 may act. Later in this book we show that such motifs do exist in arthropods and thus most presumably already in common ancestors of vertebrates and invertebrates some 600 million years ago. There are, however, in flies, snails, or shellfish additional neuropeptides endocrine-active where monobasic recognition sites are used, however, close inspection showed that most of the peptides can be cleaved at recognition sites composed of the two basic amino acids R/K spaced 0, 2, 4, or 6 amino acids apart: R/Kx n R/K (n = 0, 2, 4, 6). The lack of usual or unusual cleavage sites directly raises the question of whether peptides where an N-terminal cleavage site is missing are active as neuropeptides if at all.
In Chap. 12 we deal with circannual rhythms. In all but a few species reproduction is coupled to the annual seasons. Any activity has its time, copulation, breeding, upbringing, hibernation, or bird migration. Whereas in humans female reproductive activity cycles with a period of 28 days and humans can be sexually active on any day, in birds, for example, such behavior inhibits successful raising of the litter and would commit to much energy required, for example, for migration or survival in the cold season. In most animals reproductive activity is related to the annual cycle of seasons in males and females, at least in nondomesticated ones. A gonadotropin-inhibiting hormone in men appears dispensable, but it might definitely have a role in wild animals.
GnRH release has been previously shown to be inhibited by some neurotransmitters, however, a negative regulator had not been shown in vertebrates. Tsutsui et al. (2000) decided to look for hypothalamic peptides blocking GnRH release and identified in quail a dodecapeptide doing just that. They called this peptide gonadotropin inhibitory hormone (GnIH). Because this peptide has never been shown released into the circulation we would call the naming premature. GnIH belongs to the RFamide family of peptides present in vertebrates and invertebrates. The human GnIH homologue is cleaved from the neuropeptide-VF precursor protein.
4.3.2.2 Structure and Gene
The gene for neuropeptide VF is located on the short arm of chromosome 7. GnIH and their mammalian homologues RF-related peptides (RFRP) are characterized by the C-terminal LPxRF (x = l or Q). The human neuropeptide VF precursor contains in contrast to the chicken one only two GnIH homologues, the RFRP-2 (on blue background) has a C-terminal serine (S) instead of phenylalanine (F) (Fig. 4.7).
RFRP have unusual prohormone convertase motifs in contrast to other vertebrate hormones in this book: C-termini possess a rarely used Rx n R (n = 0, 2, 4, or 6) motif where the PC1 can act. Some singular arginines (R) might be recognized by other endopeptidases. However, inspecting the precursor sequences in this book, hormonal activity does not rely on enzymes cutting after singular arginines. Whenever these are present in a precursor, there are multiple copies of peptide sequences and there are other peptides cleaved at dibasic sites of the R/Kx n K/R with n = 0 the most obvious. Whether RFRP-1 is an active neurotransmitter is not clear and is obviously questioned by the most active group which called it only a presumable neuropeptide (Tsutsui 2009).
The GnIH/RFRP receptor is called in mammals the neuropeptide FF receptor (OT7T0222 or GPCR147) which is triggered by other RFamides, too.
In a very recent review, Tsutsui and Ubuka (2014) report an overview of GnIH in birds and mammals. They demonstrate that GnIH receptors on GnRH neurons influence the release of this hormone. What is actually blocked is the interaction of GnRHR with the Gs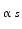 subunit, which is responsible for activating adenylate cyclase and cAMP.
subunit, which is responsible for activating adenylate cyclase and cAMP.
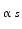 subunit, which is responsible for activating adenylate cyclase and cAMP.
subunit, which is responsible for activating adenylate cyclase and cAMP.4.3.2.3 Physiology
The original observation in quail that GnIH directly inhibits pituitary LH/FSH release (Tsutsui et al. 2000) could not be confirmed in mammals. Isolated quail pituitaries where incubated with GnIH and showed reduction of LH/FSH secretion. Whereas quail GnIH is expressed in the paraventricular nucleus (Tsutsui et al. 2000) RFRP e.g. in rats it is expressed in the dorsomedial hypothalamus (Rizwan et al. 2009. RFRP axons originating in dorsomedial hypothalamus cell bodies reach in rats to the median eminence , however, not to its external border from where neuropeptides would be released into the hypothalamic–pituitary portal system (Rizwan et al. 2009).
Inhibition of gonadotropin secretion in mammals is achieved by inhibiting not the gonadotropin releasing cells in the pituitary but by blocking the secretion of GnRH in the pituitary. RFRP influences the membrane firing of GnRH neurons, mostly inhibitory: 40 % of the investigated neurons reduced ion channel openings when treated with RFRP-3, in 10 % of these firing was enhanced and about half of neurons remained unchanged (Ducret et al. 2009).
Apart from brain GnIH/RFRP expression has also been observed in gonads. In birds (quail, chicken, starling) GnIH and its receptor GPCR147 were detected in theca and granulosa cells, in interstitial testes cells, and in the epididymis. In hamster RFRP-3 was identified in spermatocytes and spermatids together with GPCR147. There was a circannual rhythm of RFRP expression (Bentley et al. 2008).
Tsutsui and Ubuka (2014) report that by RNA interference in white-crowned sparrow, “Birds reduced resting time, spontaneous production of complex vocalizations, and stimulated brief agonistic vocalizations. GnIH RNAi further enhanced song production of short duration in male birds when they were challenged by playbacks of novel male songs. These behaviors resembled those of breeding birds during territorial defense. The overall results suggested that GnIH gene silencing induces arousal.” They have other facts about blocking influence on arousal.
GnIH and its mammalian analogues might thus fulfill a role in the circannual regulation of reproductive activity which, however, has not been sufficiently analyzed in order to give a general picture. Such regulation as mentioned above might be necessary for the survival of wildlife species. The fact that GnIH expression is induced by melatonin would fit (Ubuka et al. 2005) into the scheme, melatonin being the hormone used to estimate in molecular terms the duration of nighttime in mammals.
4.3.3 Neuropeptide Y
Neuropeptide Y (NPY ) neurons are broadly distributed a protein. The structure and functions are discussed in Sects. 4.10 and 11.5. In the hypothalamus NPY neurons localize predominantly to the arcuate nucleus. NPY release from there controls feeding and CRH release . In the periphery NPY is often formed in noradrenergic neurons .
4.3.4 Agouti-Related Protein
Fact sheet 4.6: Agouti-related
Gene:
Chromosome 16; locus 16q22; four exons
Sequence:
Synthesis and target:
AgRP is predominantly formed in the arcuate nucleus and controls feeding by binding to the melanocortin receptor 4 (MC-R4). This receptor is found on cells of the paraventricular nucleus, the dorsal motor nucleus of the vagus, and in the raphe nucleus, areas involved in energy homeostasis.
Function:
AgRP inhibits activation through MC4-R and thus permits enhanced feeding.
Receptor:
GPC heptahelical receptor: melanocortin 4 receptor.
4.3.4.1 Introduction
By discovering the Agouti gene regulation of skin pigmentation could be better understood. In humans the agouti protein is—in contrast to rodents—not restricted to the skin, but equally expressed in adipose tissue, in the testes and ovaries, in heart, and in kidney and liver (Dinulescu and Cone 2000). The agouti protein functions as an MSH antagonist at the melanocortin receptor I (MC-R1) in rodent melanocytes.
Agouti-related protein (AgRP; Fig. 4.8) has a similar antagonistic role as agouti, but at the hypothalamic MC-R4. It is involved in the regulation of feeding.
4.3.4.2 Structure and Gene
The AgRP gene was mapped to chromosome 16 (16q22). AgRP is 131 amino acids long, and its C-terminal region (82–131) is antagonistically active as is AgRP. The intramolecular cysteine bonds are functionally indispensable.
4.3.4.3 Physiology
Neurons in the arcuate nucleus produce agouti-related protein (AgRP ). This protein is a specific antagonist of the melanocortin 4 receptor MC4-R . By inhibiting MSH association with the MC4-R and thus the suppression of feeding AgRP stimulates feeding. Mice with defects of MC4-R develop gluttony and adiposity.
4.3.4.4 Phylogeny
Until now, AgRP sequences are limited to vertebrates (Klovins et al. 2004). The proteins are characterized by 10 cysteine residues. It has not been possible thus far to delineate the divergence of agouti and AgRP.
4.3.5 Somatostatin
Fact sheet 4.7: Somatostatin
Gene:
Chromosome 3; locus 3q28; two exons
Sequence:
SS14: AGCKNFFWKTFTSC
SS28: sansnpamaprerkagcknffwktftsc
(see Fig. 4.9); an intramolecular disulfide bond forms a cyclic peptide; amino acids FWK are essential for receptor binding.


Fig. 4.9
The somatostatin precursor and its derived peptides. From the precursor prosomatostatin (PSS) furin cleaves off somatostain-28 (SST-28) and SST-14 apart from a short N-terminal peptide PSS(1–10) whereas by Prohormone convertase1 only SST-14 can be released. The two cysteines by generating an intramolecular disulfide bond form the ring structure of SST (Source: GenBank NP_001039)
Synthesis and target:
Hypothalamic somatostatin is formed mainly in the paraventricular nucleus, to a lesser degree in the arcuate nucleus or ventromedial nucleus. Its targets are somatotropic cells in the pituitary and GHRH neurons in the arcuate nucleus and ventromedial nucleus. Somatostatin synthesis in the gastrointestinal tract serves regulation of endocrine cells therein, often in a paracrine way.
Function:
Somatostatin is an inhibitor of multiple endocrine functions.
Receptor:
Five human somatostatin receptors have been identified. Their cell type specific expression may explain the divergent somatostatin effects on target cells.
4.3.5.1 Introduction
The releasing hormones thus far mentioned mediate hormone secretion in the pituitary. In contrast to these hypothalamic somatostatin (SST or SRIF as somatotropin release inhibitory factor) blocks the pituitary release of the growth hormone . GH secretion is thus controlled by the balance of activating GHRH and inhibiting somatostatin. Other hypothalamic inhibitory peptides for any of the other hormones in the pituitary have not (yet?) been found, however, prolactin release is tonically suppressed by the catecholamine dopamine.
In the search for GnRH, Burgus, Ling, Butcher, and Guillemin (1973) isolated from about 500,000 ovine hypothalami a cyclic tetradecapeptide inhibiting GH release from the pituitary. At the same time they were able to report the isolation of human somatostatin.
4.3.5.2 Structure and Gene
Somatostatin is derived from a precursor by proteolytic cleavages by either PC1 or furin (Fig. 4.9). PC1 can only cleave off the short SST-14 variant , whereas furin liberates the longer SST-28 , SST-14 and an additional N-terminal peptide.
Expression of the somatostatin gene on chromosome 3 is controlled by stimulating signals increasing intracellular cAMP and by repressive influences of thus far unknown character.
4.3.5.3 Physiology
Somatostatin generation is not restricted to the hypothalamus. SST is an inhibiting agent of different endocrine and neuronal processes. In the GI tract SST attenuates multiple hormones (see Sect. 4.10); in the mammary glands milk ejection is suppressed. Apart from the hypothalamus SST neurons are located to other brain areas . Different SST functions do not originate from SST variants themselves (no functional differences found between SST-28 and SST-14). These different functions arise by differentially expressed somatostatin receptor s that mediate type-specific signal transduction pathways and are specifically expressed by cell type on various target cells (Sect. 8.2.4).
The independence of the multiple SST functions is due to the short SST half-life in blood (below 3 min) and due its rapid inactivation. For therapeutic reason an SST agonist was developed with similar SST receptor binding but an enhanced life span in blood: octreotide (Fig. 14.1).
Pituitary GH release is controlled twice by SST. SST secretion into the median eminence will inhibit GH release by SST receptor-mediated suppression of GH release in somatotropic cells. The second inhibitory signal is through direct SST action on GHRH secreting neurons still in the hypothalamus (see Müller et al. 1999).9
4.3.5.4 Phylogeny
SST is present in a variety of invertebrates. The paracrine gastrointestinal regulation from and within pancreatic islets is, however, a vertebrate achievement.
SST gene duplication: although there is a singular SST gene in the human chromosome as in other mammalian genes, fish have two different SST genes; compared to mammalian SST the product of this second SST gene has one to four amino acid exchanges (Sheridan et al. 2000).
Cortistatin: In 1996 de Lecea et al. reported another peptide with strong homology to somatostatin: cortistatin (CST), which appears to play an important role for sleep regulation. The peptide homology is 10 of 14 amino acids; all residues involved in receptor coupling are conserved (Fig. 4.10). The cysteine forming the intracellular disulfide bond and thus the cyclic peptide are conserved as well. The cortistatin gene maps to chromosome 1 (1p36.22) and comprises two exons. Three different CST peptides have been isolated: CST-14, CST-17, and CST-29.

Fig. 4.10
Cortistatin-17 and somatostatin-14: sequence comparison
CST is expressed in several tissues: in the cerebral cortex and in the hippocampus, furthermore in pancreas, gut, kidneys, testis, and leukocytes. The final proof, however, is lacking for some of these inasmuch as sometimes only the presence of RNA, but not of the CST peptide has been confirmed.
Unlike SST CST binds not only to SST receptors but the growth hormone secretagogue receptor, too (GHS-R): this receptor was first observed more than 20 years ago and has recently been identified as a ghrelin receptor. The MrgX2 (mas-related gene), first shown to mediate pain and nociception, binds CST as well (Robas et al. 2003). Proadrenomedullar peptides after binding to MrgX2 generate elevated blood pressure by inhibiting catecholamine release from sympathetic neurons or chromaffine medullary adrenal cells. In contrast to SST CST is expressed by several types of immune cells (Gonzalez-Rey et al. 2006) inhibiting endotoxin-induced cytokine release and thus protecting against lethal outcome of endotoxic shock.
In spite of these differences the endocrine functions of SST and CST are very similar with respect to central GH regulation, prolactin control, and GI-driven insulin release. The two peptides appear to be mutually restorable.
Somatostatin in invertebrates: By immunological means somatostatin or somatostatin-like peptides (already with the disulfide bond) have been found in neurons of protostomes; in deuterostomes SST was equally found in neurons, and in the gut mucosa, too: in singular neuroendocrine cells in invertebrates, however, in vertebrates in the known Langerhans islets combined with insulin (and glucagon and PNP10; Conlon et al. (1988); Falkmer et al. (1985)).
SST family in vertebrates Tostivint et al. have recently demonstrated that the genes for SST, CST, and urotensin II/urotensin-related peptide (UII/URP) arose by two gene duplications. The original precursor gave rise to a tandem of SST/CST or UII/URP genes. Such a tandem exists in very early vertebrates suggesting duplication early in development. The tandem may then be duplicated with the entire genome, an event which is timed to early fish evolution (Tostivint et al. 2006).
4.3.7 Proopiomelanocortin
POMC neurons synthesize the  -endorphine contributing to the reaction to stress as well as
-endorphine contributing to the reaction to stress as well as  -MSH which is involved in control of food uptake. Alternative processing of POMC is described in Sect. 4.4.1.
-MSH which is involved in control of food uptake. Alternative processing of POMC is described in Sect. 4.4.1.
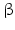 -endorphine contributing to the reaction to stress as well as
-endorphine contributing to the reaction to stress as well as 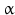 -MSH which is involved in control of food uptake. Alternative processing of POMC is described in Sect. 4.4.1.
-MSH which is involved in control of food uptake. Alternative processing of POMC is described in Sect. 4.4.1.4.3.9 Kisspeptin
Fact sheet 4.8: Kisspeptin
Gene:
Chromosome 1 (1q32); three exons
Sequence:
See Fig. 4.11
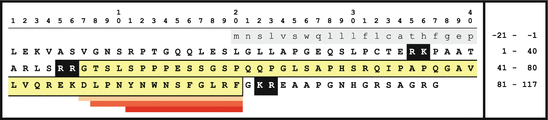

Fig. 4.11
Primary sequences of the KISS-1 protein and of derived kisspeptins. By Prohormone convertases the Kiss-1 gene product (upper case) is processed giving rise to the kisspeptin-54 yellow; after further C-terminal amidation, smaller kisspeptins 14, 13, and 10 (orange to red) are cleaved off by proteolytic digestion (Source: GenBank: NP_002247)
Synthesis and target:
Kisspeptin is formed by neurons in the arcuate nucleus and the paraventricular nucleus; it is released via synapses in the preoptic region and controls GnRH secretion.
Receptor
heptahelical GPC receptor: GPR54
4.3.9.1 Introduction
While studying tumor metastasis Lee et al. (1996) identified a protein fully blocking metastasis without inhibiting melanoma cell proliferation. GPR54 was identified as the receptor for this kisspeptin protein (Kotani et al. 2001). GPR54 knockout mice were viable but did not demonstrate sexual maturation which in turn led to kisspeptin’s role in GnRH secretion.
4.3.9.2 Structure and Genes
The KISS1 gene mapping to chromosome 1 has three exons, the first one noncoding.
Kisspeptins (Fig. 4.11) are derived from the primary KISS-1 protein by posttranslational modifications. Aside from kisspeptin 54 the literature reports smaller kisspeptins with chain length of 10 to 14 amino acids. The peptidase for tissue-specific processing is not yet identified.
4.3.9.3 Physiology
Kisspeptins supposedly have two roles: they block metastases of tumor as well as of placenta cells and, centrally, they control GnRH release in the median eminence :
1.
Metastasis inhibiting function: From the first description on, the number of reports on suppression of invasive tumor migration has been ever increasing. In some tumors suppression of NF-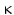 B translocation into the nucleus by kisspeptin was shown. In addition different signal pathways via protein kinase A or protein kinase C were shown. Blocking metastasis may be related to CXCR4 signal transduction; CXCR4 is seen as an important player in metastasis and in the interactions of cells with the environment (Navenot et al. 2005). In mice Bilban et al. (2004) have shown that kisspeptin and its receptor regulating trophoblast invasion into the maternal endometrium are predominantly expressed in early gestation: at term only the measurable Kiss RNA was 30 times less than in the third month of gestation.
B translocation into the nucleus by kisspeptin was shown. In addition different signal pathways via protein kinase A or protein kinase C were shown. Blocking metastasis may be related to CXCR4 signal transduction; CXCR4 is seen as an important player in metastasis and in the interactions of cells with the environment (Navenot et al. 2005). In mice Bilban et al. (2004) have shown that kisspeptin and its receptor regulating trophoblast invasion into the maternal endometrium are predominantly expressed in early gestation: at term only the measurable Kiss RNA was 30 times less than in the third month of gestation.
 B translocation into the nucleus by kisspeptin was shown. In addition different signal pathways via protein kinase A or protein kinase C were shown. Blocking metastasis may be related to CXCR4 signal transduction; CXCR4 is seen as an important player in metastasis and in the interactions of cells with the environment (Navenot et al. 2005). In mice Bilban et al. (2004) have shown that kisspeptin and its receptor regulating trophoblast invasion into the maternal endometrium are predominantly expressed in early gestation: at term only the measurable Kiss RNA was 30 times less than in the third month of gestation.
B translocation into the nucleus by kisspeptin was shown. In addition different signal pathways via protein kinase A or protein kinase C were shown. Blocking metastasis may be related to CXCR4 signal transduction; CXCR4 is seen as an important player in metastasis and in the interactions of cells with the environment (Navenot et al. 2005). In mice Bilban et al. (2004) have shown that kisspeptin and its receptor regulating trophoblast invasion into the maternal endometrium are predominantly expressed in early gestation: at term only the measurable Kiss RNA was 30 times less than in the third month of gestation.2.
Regulation of GnRH release: Kisspeptin is expressed in neurons of the arcuate nucleus and released in the paraventricular nucleus and the preoptic nuclei. Estradiol is known to control kisspeptin release; whether GABA or further neurotransmitters modulate kisspeptin secretion is thus far unknown. The control of the pulsatile secretion is further unknown. Neurons in the paraventricular nucleus are at least involved in the preovulatory LH peak; the regulation of this is not known either in women nor in any animal.
4.3.9.4 Phylogeny
Thus far kisspeptins and the kisspeptin receptor are found in vertebrates: primates, rodents, fish. RFamides, however, are among the primordial neuropeptides and found in those species with the earliest existence of neurons and neurosecretion.
Similarly kisspeptin during maturation of gonads was observed in fish as in man: male D. rerio express kisspeptin maximally during the first formation of sperm.
4.3.10 Galanin
Fact sheet 4.9: Galanin
Gene:
Chromosome 11; locus 11q13.2; six exons
Sequence:
see Fig. 4.12


Fig. 4.12
The galanin preproproteins: after removal of the signal peptide(upper line) the galanin peptide (in uppercase and blue) is liberated from the proprotein by Prohormone convertase1. Dibasic peptide motifs are shown inversely. An alternative galanin-related peptide lacks the first Prohormone convertase1 motif; additional spaces are added to superimpose the homologous partial galanin sequence and the second KR motif (Source: GenBank CAA01907 and NP_149097)
Synthesis and target:
Galanin is released from CNS neurons, from neurons of the enteric nerve system, as well as by cytotrophoblastic cells of the placenta, and acts in endocrine and paracrine fashions on cells regulating feeding, insulin release, memory, and reproduction.
Function:
Galanin is a regulator of different aspects of homeostasis and of reproduction. Its presence is essential for maturation of mammary glands and of milk synthesis.
Receptor:
The three human galanin receptors are heptahelical GPC membrane receptors.
4.3.10.1 Introduction
Galanin was first identified as a gut peptide (Tatemoto et al. 1983). Later on it was shown to be expressed in multiple types of neurons. Its expression in the human placenta could also be shown (Kleine et al. 2001). Recent literature demonstrates divergent roles of galanin.11 Experimentally proven is a direct relationship to uptake of a fatty enriched diet. The indispensable role of galanin for mammary gland maturation and function has already been shown before.
4.3.10.2 Structure and Genes
The preprotein is transcribed from the gene on chromosome 11 (close to a metalloproteinase gene). After removal of the signal peptide the PC1 cuts galanin from the proprotein. In animals the terminal glycine is amidated. Due to an amino acid change from glycine to serine, nonamidated human galanin remains and its sequence is prolonged by one amino acid.
First in swine and then in other mammals an alternative galanin-related peptide (GalrP) was found. The human GalrP lacks the first PC1 motif. The sequence identical to galanin is thus N-terminally elongated (Fig. 4.12).
Three galanin receptors belong to GPC heptahelical membrane receptor families. Gal-R1 and Gal-R3 induce adenylate cyclase and thus cAMP elevation, and Gal-R2 signals via phospholipase C.
4.3.10.3 Physiology
Experiments in mice have demonstrated galanin’s functions in at least two pathways: galanin-defective (Gal  ) mice were viable and fertile which precludes any essential role in reproduction. However, once these Gal
) mice were viable and fertile which precludes any essential role in reproduction. However, once these Gal  females had given birth they were unable to feed their offspring because the mammary glands were afunctional. Gal
females had given birth they were unable to feed their offspring because the mammary glands were afunctional. Gal  mice utilized a fat-enriched diet to a lesser extent than comparable wildtype mice. Whether the phenomena are important in humans is not yet proven.
mice utilized a fat-enriched diet to a lesser extent than comparable wildtype mice. Whether the phenomena are important in humans is not yet proven.
 ) mice were viable and fertile which precludes any essential role in reproduction. However, once these Gal
) mice were viable and fertile which precludes any essential role in reproduction. However, once these Gal  females had given birth they were unable to feed their offspring because the mammary glands were afunctional. Gal
females had given birth they were unable to feed their offspring because the mammary glands were afunctional. Gal  mice utilized a fat-enriched diet to a lesser extent than comparable wildtype mice. Whether the phenomena are important in humans is not yet proven.
mice utilized a fat-enriched diet to a lesser extent than comparable wildtype mice. Whether the phenomena are important in humans is not yet proven.In addition, Gal  mice do not show estradiol-induced prolactin stimulation. Thus, galanin appears to be an estrogen-dependent stimulator of pituitary prolactin synthesis and release. Galanin receptor mutations may be factors of prolactinoma development.
mice do not show estradiol-induced prolactin stimulation. Thus, galanin appears to be an estrogen-dependent stimulator of pituitary prolactin synthesis and release. Galanin receptor mutations may be factors of prolactinoma development.
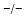 mice do not show estradiol-induced prolactin stimulation. Thus, galanin appears to be an estrogen-dependent stimulator of pituitary prolactin synthesis and release. Galanin receptor mutations may be factors of prolactinoma development.
mice do not show estradiol-induced prolactin stimulation. Thus, galanin appears to be an estrogen-dependent stimulator of pituitary prolactin synthesis and release. Galanin receptor mutations may be factors of prolactinoma development.Neurons exclusively forming galanin may not exist: galanin has been co-localized to neurons and cells expressing multiple other hormones or neurotransmitters: GnRH, GHRH, prolactin, vasopressin, CRH, oxytocin, substance P, CGRP, noradrenaline, or acetylcholine. Using patch clamp techniques, galanin was shown to inhibit neurons by elevating potassium currents (triggered by Gal-R1 and Gal-R3) and by downregulating calcium currents. Galanin also blocks synaptic plasticity, for example, when memory develops (long-term potentiation). Especially in the arcuate nucleus galanin presynaptically reduces GABA release as well as postsynaptically via galanin receptors. Singular effects by galanin stimulation have been observed in the dorsal–vagal complex where calcium currents were decreased.
Apart from the CNS is galanin expressed in the anterior pituitary, in the adrenal medulla, in the pancreas, the urogenital tract, and in skin (Wynick and Bacon 2002; Tortorella et al. 2007; Bauer et al. 2008). Neurons innervating the heart, kidneys, or gut have also been found to be galanin positive by immunocytochemistry.
When axons are cut, galanin and its receptors are upregulated. Galanin also seems relevant for nociception and neuronal development in spinal ganglia in mice (Hobson et al. 2008). Animal models suggest effects of galanin on learning and memory (Miller 1998; Rustay et al. 2005).
Galanin might also play a role during reproduction: it is hypothalamically co-expressed together with GnRH; the count of galanin plus GnRH double positive neurons was fivefold elevated in female rats compared to male. This difference may be due to a testis-dependent epigenetic regulation (Merchenthaler 1998).
Most recent articles suggest that galanin characterizes metastatic breast cell tumors. Further such markers are vascular endothelial growth factor (VEGF) and related drugs induced during tissue hypoxia by hypoxia-induced factor 1 (HIF1). Given that galanin is required for mammary gland maturation such a role is feasible (Bertucci and Birnbaum 2009).
4.3.10.4 Phylogeny
Galanin has been identified thus far in vertebrates.12 However, proteins with homology have been found in placozoans, that is, before protostomes and deuterostomes developed separately. Allatostatin receptors in insects share up to 50 % homology with vertebrate galanin receptors.
4.3.11 Melanin-Concentrating Hormone
Fact sheet 4.10: Melanin concentrating hormone (MCH)
Gene:
Chromosome: 12; locus: 12q23.2; three exons
Sequence:
DFDMLRCMLGRVYRPCWQV (Fig. 4.13).


Fig. 4.13
Primary sequence of human melanin concentrating hormone (MCH). The signal peptide is highlighted light gray, the MCH peptide yellow and two other peptides dark gray. Endopeptidase motifs are inverted. The disulfide bridge of MCH is indicated by blue lines (Source: GenBank NM_002665.2)
Synthesis and target:
MCH is released from the lateral hypothalamic area and acts on MCH receptors in brain and in the periphery.
Function:
Mammalian MCH is a neuropeptide regulator of feeding behavior and of energy expenditure; in fish and amphibia it is active as a melanophore-concentrating hormone and triggers lightening of the skin.
Receptor:
Two GPCR MCH-R1 (aka orphan receptor SLC-1) and MCH-R2 with different functional and topological characteristics.
4.3.11.1 Introduction
The adaption of the fish skin to the environment expressed as darkening or lightening has been suggested to be controlled by mutually antagonistic peptides since the year 1931 (Hogben and Slome 1931). The hormone for darkening turned out to be the MSH (from POMC), however, a melanin-concentrating hormone could only be identified in 1983 by Kawauchi et al. (1983) in salmon. A homologous hormone was later isolated and cloned from different mammals including humans. Several mammalian MCH share the same structure; in mice there are two amino acid exchanges and in sheep the sequence appears truncated (Pissios et al. 2006).
4.3.11.2 Structure and Gene
The original MCH from salmon is a 17 amino acid long cyclic peptide with an intramolecular disulfide bridge ; human (and common mammalian) MCH has two additional N-terminal amino acids and few exchanges compared to the salmon sequence. The peptide is released from a precursor which in addition to the signal peptide and the MCH bears two other peptides called neuropeptide GE and neuropeptide EI (due to the termini), the latter being amidated (Fig. 4.13). The human MCH gene on chromosome 12 has three exons; the next neighbors are a nucleoporin and IGF1.
The MCH receptor was identified to be the orphan GPCR SLC-1 which was relabeled to MCH-R1. The receptor couples to different G- proteins and activates different signaling pathways: increase in intracellular free calcium, suppression of forskolin stimulated cAMP, stimulation of phophoinositol pathways, and triggering of extracellular-signal-regulated kinases (ERK). The receptor is preferentially expressed in the brain, highly in the piriform cortex and the olfactory tubercle, and with lower density in the nucleus accumbens and the amygdala. Further expression has been found in the arcuate nucleus and the ventromedial nucleus.
By Northern blot and in situ hybridization analysis of human and monkey tissue, Sailer et al. (2001) showed that expression of MCH-R2 mRNA is restricted to several regions of the brain, including the arcuate nucleus and the ventral medial hypothalamus, areas implicated in regulation of body weight.
4.3.11.3 Physiology
The original function in fish (lightening of skin) has been lost during further vertebrate development. Today human MCH is considered an important regulator of food intake and energy expenditure. This has been shown by different authors, either in model animals or in humans (for reviews, see Nahon 2006; Flier 2004; Pissios et al. 2006). The first hint for this event stems from direct intracerebral injections of MCH into rat brains (Qu et al. 1996) which induced food intake. Careful analysis by a couple of laboratories identified the lateral hypothalamic area (LHA) as the place where most MCH perikarya were located with projections into many other brain areas. This is conclusive for a neurotransmitter role of MCH in induction of feeding and energy expenditure. The LHA, in addition, comprises another type of cell directly related to appetite and feeding behavior: orexin neurons and orexins likewise active as feeding control neurotransmitters.
Whereas POMC and other neurons almost always show a membrane potential, MCH neurons are mostly quiet and become active upon stimulation, either by synaptic contacts or, as shown recently, by elevated glucose levels. Upon stimulation MCH neurons secrete MCH over synapses and, to some lower degree, into the circulation. The role of MCH in periphery, however, has not attracted attention as do the neurotransmitter actions, and it thus largely speculative. Skin cells and some other cell types have been shown to express MCH-R1.
Most instructive have been MCH knockout mice: These mice were hypophagic (reduced food intake) and lean compared to wildtype littermates. This leanness was attributed to an enhanced energy consumption in these MCH-deficient mice.
Long-term exposure to intracerebroventricular MCH led to an increased body weight in mice, which increased even further with a parallel 33 % fat diet (one fifth compared with 1/20 (with normal diet) within 14 days of MCH exposure). Rats kept in the cold appear to depend on MHC to adapt to the low temperature by activating brown adipose tissue’s fat consumption: blocking MHC expression by antisense RNA resulted in a dramatic weight loss compared to controls. Using an MCH receptor antagonist it could be shown in obese mice that this treatment reduced food uptake, stopped body weight gain, instead reduced body weight, lowered the overall fat content, and reduced hypercholesterolemia, hyperinsulinemia, hyperglycemia, and hyperleptinemia associated with obesity in these animals.
These facts demonstrate clearly a neuropeptide control of MCH on feeding, energy mobilization, and accumulation of energy stores.
The interaction of MCH neurons with other central centers of feeding are dealt with in the chapter about feeding (Sect. 11.5.8).
4.3.11.4 Phylogeny
Any MCH-like protein has only been described in vertebrates.
4.3.12 Orexins
Fact sheet 4.11: Orexins/Hypocretin (HCRT)
Gene:
Chromosome 17; locus 17q21; two exons.
Synthesis and target:
Two orexins, A and B, cleaved from the same precursor; orexins are formed in neurons (neurosecretory cells?) of the lateral hypothalamus and the enteric nervous system (ENS) and almost exclusively released as neurotransmitters.
Function:
Orexins are involved in regulation of feeding and the maintenance of a regular sleep cycle.
Receptor:
Two heptahelical GPCR, HCRT-R1 and HCRT-R2; HCRT-R1 is recognized by orexin-A, whereas HCRT-R2 is bound by both orexins.
4.3.12.1 Introduction
Orexins, also known as hypocretins (i.e., incretins of the hypothalamus, belonging to the secretin protein family), were identified in 1998 as peptides contributing to the regulation of feeding (de Lecea et al. 1998). In the meantime it has been additionally found that they play a role in the regulation of sleep because a defect in the orexin gen triggers familiar narcolepsy, and acquired narcolepsy can be induced by destruction of orexin neurons in the lateral hypothalamus. The original assumption of an exclusive presence of orexin neurons in the brain has been discarded when orexin formation could also be observed in the enteric nerve system (ENS) (Kirchgessner 2002).
4.3.12.2 Structure and Gene
The gene for the orexin precursor has been found on the long arm of chromosome 17. After removal of the 33 amino acids of the signal peptide orexin A is released by PC1 and orexin 2 by PC2. Therefore the release of orexin B is coupled to the expression of the PC2. The extent of expression of this enzyme in the lateral hypothalamus and its regulation in the brain region has not yet been conclusively analyzed. Other prohormone convertase—for example, furin—do not play any role due to the lack of peptide motifs in the orexin preprotein.
Orexin-A contains two N-terminal disulfide bridges missing in orexin-B. This difference is already present in fish e.g. ABF29871.1 from codfish. The two peptides are C-terminally amidated; orexin-A has an N-terminal pyroglutamate (pE; Fig. 4.15). These peptides have been analyzed by NMR (Fig. 4.16). The C-terminal helices share structural homology; the sequence homology is enhanced in the C-terminal region compared to the N-terminus.

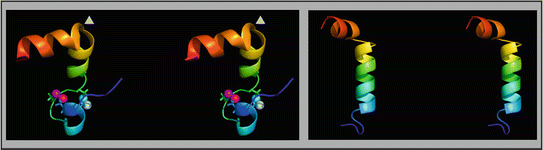


Fig. 4.14
Orexin precursor and its primary sequence. Following the 33 amino acids of the signal peptide (highlighted gray) there are two orexin peptides: orexin A (on a yellow background) harboring two disulfide bridges (lines connecting blue cysteines) and the cysteine lacking orexin B (on orange) (Source Swiss-Prot: O43612.1.; PyMOL)
Fig. 4.15
Structures of the human orexins. The stereo models of orexin A (left) and orexin B (right) show a blue colored N-terminus (in the lower part of the image) and a red colored C-terminus. The arrowheads in the orexin A image indicate a  -turn
-turn
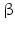 -turn
-turn
Fig. 4.16
Homology of orexins and secretin. The homology of both orexins is highest in those helices Kim et al. (2004a) determined. Also the homology to the eponymous secretin is obvious (compare the fact sheet 4.36). Orexins are indeed the secretine of the hypothalamus. This homology is mostly due to the helices Doppelmarkierung above the orexin-A sequence. The first eight amino acids of secretin are homologous to the C-teminal sequence of both orexins
Two heptahelical GPCR have been shown to be orexin receptors. Their sequence homology is 64 %. The HCRT-R1 activates upon ligand binding hypoxia-inducible factor (HIF-1a). This HIF-1a activation leads to increased glucose uptake and enhanced glycolysis. In contrast to the hypoxia-induced activation ATP is not formed by anaerobic glycolysis but by stimulating the citrate pathway and oxidative phosphorylation (Sikder and Kodadek 2007). HRCT-2 is linked in dogs to inherited narcolepsy (Lin et al. 1999): an insertion of 226 base pairs in intron 3 of the HCRT-2 gene causes aberrant splicing and a shortened and afunctional receptor protein.
4.3.12.3 Physiology
In the brain orexin neurons are almost exclusively found in the lateral hypothalamus which is among others a center for the regulation of feeding. The orexin neurons of rat project their axon in different brain regions (Hagan et al. 1999): the lateral hypothalamus itself, the perifornical nucleus, the dorsal raphe nucleus, the periaqueductal gray, as well as into the paraventricular and centromedial nuclei of the thalamus. Most intensively orexin neuron axon were found in the locus coeruleus. Here mostly probably noradrenergic neurons are synaptically connected to orexin-A. The neurons in the LC13 are especially involved in the regulation of stress (see Sect. 11.2.1) and in the control of alertness and sleep. Orexin intracerebroventricularly injected prolonged states of alertness, enhanced locomotor activity, and reduced sleep periods. There are related findings that lack of orexin or a defective orexin receptor are at the origin of narcolepsy (Lin et al. 1999; Chemelli et al. 1999; Ebrahim et al. 2003, 2005). Orexin is thus applied when alertness should be enhanced.
Orexin positive cells are additionally found in the enteric nervous system in the mucosa of humans and animals: in the plexus myentericus and the plexus submucosus about one of four cells was orexin and leptin positive (Kirchgessner and Liu 1999). The authors suggest, therefore, that orexins have an important role in energy homeostasis.
4.3.12.4 Phylogeny
Thus far orexin sequences have been found in vertebrates and lampreys. In the latter case (XP_002598524) an orexin is not formed when a PC2 is active, only fragments thereof. In skate (Leucoraja ocellata) two orexins are coded for, disulfated bridges are present, and intramolecular PC2 motifs are absent. In the codfish Xu and Volkoff (2007) there are two peptides, 50 and 29 amino acids long, released by PC1. In humans, only the orexin-A is released by the PC1, not orexin-B, which raises doubts about its role as a neuropeptide, and both peptides can be targeted by the PC2 (see Fig. 4.14).
4.4 Anterior Pituitary Hormones
4.4.1 POMC
Fact sheet 4.12: Proopiomelanocortin (POMC)
Gene:
Chromosome 2; locus: 2p23; three or four exons.
Synthesis and target:
The prohormone is formed in corticotropic cells of the anterior pituitary, within melanotropic cells of the pars intermedia, in the hypothalamus and in skin. Due to the expression of PC1 and/or PC2 ACTH, β-endorphins and/or MSH peptides are synthesized.
Receptor:
Heptahelical G protein-coupled receptors: melanocortin receptors, opioid receptors.

Introduction
Proopiomelanocortin (POMC ) constitutes the precursor of seven different peptide hormones. It is expressed predominantly in corticotropic cells of the adenohypophysis as well as in melanotropic cells of the pars intermedia which in adult life is regressed. After processing POMC in the anterior pituitary adrenocorticotropic hormone (ACTH ), 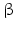 -lipotropin (LPH ) as well as
-lipotropin (LPH ) as well as 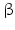 -endorphin are stored in vesicles. In contrast, in the intermediate lobe melanocortins (MSH ) and acetyl-
-endorphin are stored in vesicles. In contrast, in the intermediate lobe melanocortins (MSH ) and acetyl-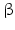 -endorphin are formed. POMC is further expressed in the hypothalamus , in the testis , in the ovary , in the adrenal medulla , in placenta , in the lungs , in skin , and especially in circulating monocytes and in tissue macrophages .
-endorphin are formed. POMC is further expressed in the hypothalamus , in the testis , in the ovary , in the adrenal medulla , in placenta , in the lungs , in skin , and especially in circulating monocytes and in tissue macrophages .
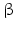 -lipotropin (LPH ) as well as
-lipotropin (LPH ) as well as 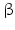 -endorphin are stored in vesicles. In contrast, in the intermediate lobe melanocortins (MSH ) and acetyl-
-endorphin are stored in vesicles. In contrast, in the intermediate lobe melanocortins (MSH ) and acetyl-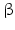 -endorphin are formed. POMC is further expressed in the hypothalamus , in the testis , in the ovary , in the adrenal medulla , in placenta , in the lungs , in skin , and especially in circulating monocytes and in tissue macrophages .
-endorphin are formed. POMC is further expressed in the hypothalamus , in the testis , in the ovary , in the adrenal medulla , in placenta , in the lungs , in skin , and especially in circulating monocytes and in tissue macrophages .Structure and Genes
The POMC gene on chromosome 2 gives rise to two variants differing by an additional exon of 50 bp in the 5′ untranslated region. The POMC mRNA in pituitary and hypothalamus is about 1.1 kilobases (kB) long, whereas extracranial RNA has only 800–900 bp. In tumors there is a further RNA of about 1.4 kB size.
From the different POMC RNAs the same precursor protein is translated: POMC with 267 amino acids in humans. From this precursor, different fragments are cleaved by PC1/PC2. Three different types of peptides are known: melanocortins, adrenocorticotropic hormone (corticotropin, ACTH), and endorphins (see Fig. 4.17). These peptide hormones have different action. Which neuroendocrine cells which of these hormones releases is determined by the expression of the prohormone convertases. In addition, from bovine POMC it is known that different glycosylations influence the use of the peptide motifs by the prohormone convertases (Birch et al. 1991).
The POMC gene is predominantly expressed in corticotropic cells of the pituitary, in neurons of the arcuate nucleus and the nucleus of the solitary tract, and in keratinocytes and melanocytes of the skin.
The dog sequence shown in Fig. 4.17 has been retracted from GenBank due to low quality. Another sequence (XP_849463) still shows the KK motif (at the end of the middle block) which indicates that ACTH may not be a product from the anterior pituitary in dog, swine, opossum, chicken, and frog because the PC1 motif is lacking. In fish, however, there are two POMC genes and only the one shown of zebrafish has mutation translated into the KR to KK change. The gene product of the second gene can be processed to give rise to ACTH (Alsop and Vijayan 2009).
The corticotropic cells of the pituitary express predominantly PC1 forming thus ACTH , 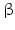 -Lipotropin and
-Lipotropin and 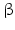 -Endorphin from POMC. In contrast, melanocytes of the intermediate lobe express additional PC2 which in turn forces
-Endorphin from POMC. In contrast, melanocytes of the intermediate lobe express additional PC2 which in turn forces 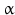 -MSH synthesis. POMC neurons of the ventral hypothalamus equally synthesize
-MSH synthesis. POMC neurons of the ventral hypothalamus equally synthesize 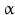 -MSH.
-MSH.
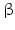 -Lipotropin and
-Lipotropin and 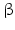 -Endorphin from POMC. In contrast, melanocytes of the intermediate lobe express additional PC2 which in turn forces
-Endorphin from POMC. In contrast, melanocytes of the intermediate lobe express additional PC2 which in turn forces 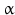 -MSH synthesis. POMC neurons of the ventral hypothalamus equally synthesize
-MSH synthesis. POMC neurons of the ventral hypothalamus equally synthesize 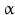 -MSH.
-MSH.Phylogeny
POMC and the peptides derived thereof have thus far been found but in vertebrates and agnathans. The genome of the urochordate S. purpurata 14 contains neither a POMC precursor nor any melanocortin receptor (Burke et al. 2006). There are, however, reports that parasitic Schistosoma mansoni expresses POMC as a defense against vertebrate immune attack and releases MSH, ACTH, and 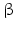 -endorphin peptides. Since these reports in 1992 (Duvaux-Miret et al. 1992), a POMC gene from S. mansoni has not been published. Furthermore, an isolation of
-endorphin peptides. Since these reports in 1992 (Duvaux-Miret et al. 1992), a POMC gene from S. mansoni has not been published. Furthermore, an isolation of 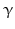 -MSH from leech has been reported. In the blue mussel POMC derived peptides were reported as well. Again any genomic sequence has yet to be found.
-MSH from leech has been reported. In the blue mussel POMC derived peptides were reported as well. Again any genomic sequence has yet to be found.
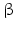 -endorphin peptides. Since these reports in 1992 (Duvaux-Miret et al. 1992), a POMC gene from S. mansoni has not been published. Furthermore, an isolation of
-endorphin peptides. Since these reports in 1992 (Duvaux-Miret et al. 1992), a POMC gene from S. mansoni has not been published. Furthermore, an isolation of 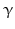 -MSH from leech has been reported. In the blue mussel POMC derived peptides were reported as well. Again any genomic sequence has yet to be found.
-MSH from leech has been reported. In the blue mussel POMC derived peptides were reported as well. Again any genomic sequence has yet to be found.In most vertebrates a single POMC exists; a second POMC gene exists in fish after an additional genome duplication (de Souza et al. 2005). Fish POMCα is expressed in the hypothalamic nucleus lateralis tuberis, in the anterior pituitary, and in the intermediate lobe, whereas POMC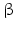 is found in the preoptic region and faintly in the pituitary stalk. The endorphin sequence is retained only in POMC
is found in the preoptic region and faintly in the pituitary stalk. The endorphin sequence is retained only in POMC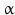 .
.
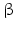 is found in the preoptic region and faintly in the pituitary stalk. The endorphin sequence is retained only in POMC
is found in the preoptic region and faintly in the pituitary stalk. The endorphin sequence is retained only in POMC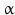 .
.Comparing selected mammalian and other vertebrate POMC sequences (Fig. 4.17), you may notice that:
The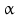 -MSH sequence is conserved from teleosts on; there are two amino acid exchanges in clawed frog and in cows. The dibasic sequence motifs are conserved, too. Apart from X. laevis the glycines for C-terminal amidation and the N-terminal serine that is acetylated are always present.
-MSH sequence is conserved from teleosts on; there are two amino acid exchanges in clawed frog and in cows. The dibasic sequence motifs are conserved, too. Apart from X. laevis the glycines for C-terminal amidation and the N-terminal serine that is acetylated are always present.
In rats and mice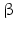 -MSH is lacking because the KK-cleavage site is gone.
-MSH is lacking because the KK-cleavage site is gone.
Teleosts do not have a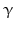 -MSH.
-MSH.
Within mammals ACTH has two characteristic exchanges in rodents. Nonmammalian ACTH have some constant differences compared to mammals. The lack of a PC1 motif in dog, swine, opossum, or chicken raises the question whether and how these animals make ACTH. There is no report in the literature on the problem. In D. rerio a second POMC rescues this defect, however, in tetrapods and birds only a single gene has ever been reported.
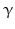 -Lipocortin is the most variant part of the different POMC regions.
-Lipocortin is the most variant part of the different POMC regions.
Most interesting is the high conservation of the N-terminal peptide: the four cysteines are at similar positions and equally spaced. With the exception of opossum there are but two positions with alternative amino acids although any function of this sequence has not yet been described.
The melanocortin receptors reflect the different genome duplications (Fig. 4.18). In lamprey i.e. before the second genome duplication two MCR were found. After the next genome duplication vertebrates have five MCR genes which in humans are on chromosomes 16 and 18 with synteny tom, for example, fugu (Klovins et al. 2004). After the third genome duplication restricted to fish in the thus far analyzed species several genes got lost including both the MCR3 genes in Takifugu rubripes as well as the 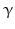 -MSH (as shown in fugu, zebrafish, and trout). MCR3 is the specific receptor for
-MSH (as shown in fugu, zebrafish, and trout). MCR3 is the specific receptor for 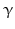 -MSH in humans and other species analyzed.
-MSH in humans and other species analyzed.
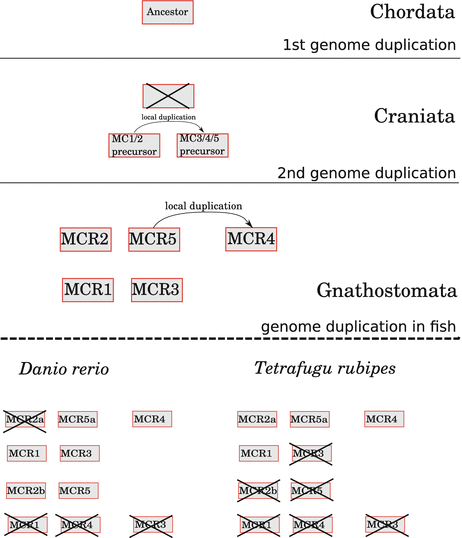
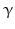 -MSH (as shown in fugu, zebrafish, and trout). MCR3 is the specific receptor for
-MSH (as shown in fugu, zebrafish, and trout). MCR3 is the specific receptor for 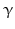 -MSH in humans and other species analyzed.
-MSH in humans and other species analyzed.
Fig. 4.18
Hypothesis of melanocortin receptor gene (MCR) development. After an initial genome duplication of an unknown ancestor gene one gene is lost and the other locally duplicated again. A second genome duplication was followed by doubling of one of the resulting genes. The pattern of five MCR is found in the majority of vertebrates. In fish a third genome duplication took place. Not all of the genes were maintained which gave rise to characteristic patterns like those of D. rerio or T. rubripes (Source: redrawn due to Klovins et al. 2004)
The topological distribution of the hormone targets merits attendance.
In humans melanocortin receptor 1 (MCR1) is expressed in skin: in melanocytes, keratinocytes, fibroblasts, endothelial cells, and antigen presenting cells. This receptor binds 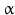 -MSH and ACTH with equal specificity. In leukocytes MCR1 mediates the inflammation inhibiting effects of
-MSH and ACTH with equal specificity. In leukocytes MCR1 mediates the inflammation inhibiting effects of 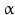 -MSH. MCR2 is the receptor in the adrenal cortex mediating the steroidogenic activity of ACTH. This receptor is not activated by MSHs. MCR3 is expressed in CNS, gastrointestinal tract, and in placenta.
-MSH. MCR2 is the receptor in the adrenal cortex mediating the steroidogenic activity of ACTH. This receptor is not activated by MSHs. MCR3 is expressed in CNS, gastrointestinal tract, and in placenta. 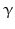 -MSH exhibits the highest activity for this receptor. MCR4 is specially expressed in CNS.
-MSH exhibits the highest activity for this receptor. MCR4 is specially expressed in CNS. 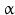 -MSH and ACTH activate this receptor more strongly than
-MSH and ACTH activate this receptor more strongly than 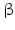 -MSH or
-MSH or 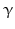 -MSH. Finally, MCR5 is the
-MSH. Finally, MCR5 is the 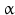 -MSH receptor of sebaceous glands but found in other tissues, too. Obviously the receptor has its role in the regulation of exocrine glands.
-MSH receptor of sebaceous glands but found in other tissues, too. Obviously the receptor has its role in the regulation of exocrine glands.
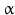 -MSH and ACTH with equal specificity. In leukocytes MCR1 mediates the inflammation inhibiting effects of
-MSH and ACTH with equal specificity. In leukocytes MCR1 mediates the inflammation inhibiting effects of 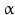 -MSH. MCR2 is the receptor in the adrenal cortex mediating the steroidogenic activity of ACTH. This receptor is not activated by MSHs. MCR3 is expressed in CNS, gastrointestinal tract, and in placenta.
-MSH. MCR2 is the receptor in the adrenal cortex mediating the steroidogenic activity of ACTH. This receptor is not activated by MSHs. MCR3 is expressed in CNS, gastrointestinal tract, and in placenta. 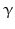 -MSH exhibits the highest activity for this receptor. MCR4 is specially expressed in CNS.
-MSH exhibits the highest activity for this receptor. MCR4 is specially expressed in CNS. 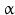 -MSH and ACTH activate this receptor more strongly than
-MSH and ACTH activate this receptor more strongly than 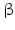 -MSH or
-MSH or 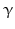 -MSH. Finally, MCR5 is the
-MSH. Finally, MCR5 is the 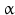 -MSH receptor of sebaceous glands but found in other tissues, too. Obviously the receptor has its role in the regulation of exocrine glands.
-MSH receptor of sebaceous glands but found in other tissues, too. Obviously the receptor has its role in the regulation of exocrine glands.Compared with humans, the distribution and functional specificity in T. rubripes is different: truMcR1 is faintly expressed but in CNS; truMcR2 in brain and in the adrenal, (truMcR3 is lacking); truMcR4 and truMcR5 are found in brain, in the adrenal, and in gut (truMcR4), respectively; and in the eye (truMcR5). As in humans in T. rubipes truMcR2 is stimulated exclusively by ACTH, not by any MSH. truMcR1 and truMcR4 show much stronger activation by ACTH than by 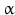 -MSH (Klovins et al. 2004): this is different from humans and interpreted by the authors as a hint to an ancestral function of ACTH.
-MSH (Klovins et al. 2004): this is different from humans and interpreted by the authors as a hint to an ancestral function of ACTH.
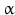 -MSH (Klovins et al. 2004): this is different from humans and interpreted by the authors as a hint to an ancestral function of ACTH.
-MSH (Klovins et al. 2004): this is different from humans and interpreted by the authors as a hint to an ancestral function of ACTH.4.4.1.1 ACTH
Fact sheet 4.13: Adrenocorticotropic hormone (ACTH)
Sequence:
SYSMEHFRWG KPVGKKRRPV KVYPNGAEDE SAEAFPLEI
Synthesis and target:
In POMC forming cells by activity of prohormone convertase 1
Function:
Stimulation of steroidogenesis in the adrenal
Receptor:
Heptahelical G-protein-coupled receptor: melanocortin 2 receptor
Introduction
ACTH is the effector hormone of the HPA axis and stimulates hormone release in the adrenals: glucocorticoids as well as mineralocorticoids are released after ACTH stimulation, as well as adrenaline whose synthesis is stimulated by ACTH which induces the last enzyme of adrenaline synthesis: PNMT (see Fig. 7.1).
Physiology
ACTH is released from adrenocorticotropic cells in the pituitary by hourly pulses. This secretion thus results from hypothalamic CRH release into the median eminence and into the hypophyseal portal system. During night, the quantity of ACTH is twice to thrice as high as during daytime (see Fig. 12.2). ACTH stimulates by its effect on the adrenal MCR2 receptors intracellular cAMP resulting in steroid hormone synthesis and (because these are not stored in granules) release. Of paramount importance is the cortisol formation.
Regulation of POMC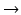 ACTH release in the pituitary results from stimulation of intracellular cAMP by CRH and binding of transcription factor Nur to Nur-responsive elements in the POMC promoter. Feedback inhibition by glucocorticoids is due to direct interaction of ligand-bound glucocorticoid receptors with Nur, thus blocking its interaction with DNA (Murakami et al. 2007).
ACTH release in the pituitary results from stimulation of intracellular cAMP by CRH and binding of transcription factor Nur to Nur-responsive elements in the POMC promoter. Feedback inhibition by glucocorticoids is due to direct interaction of ligand-bound glucocorticoid receptors with Nur, thus blocking its interaction with DNA (Murakami et al. 2007).
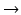 ACTH release in the pituitary results from stimulation of intracellular cAMP by CRH and binding of transcription factor Nur to Nur-responsive elements in the POMC promoter. Feedback inhibition by glucocorticoids is due to direct interaction of ligand-bound glucocorticoid receptors with Nur, thus blocking its interaction with DNA (Murakami et al. 2007).
ACTH release in the pituitary results from stimulation of intracellular cAMP by CRH and binding of transcription factor Nur to Nur-responsive elements in the POMC promoter. Feedback inhibition by glucocorticoids is due to direct interaction of ligand-bound glucocorticoid receptors with Nur, thus blocking its interaction with DNA (Murakami et al. 2007).ACTH’s half-life in human blood was determined to be 19 min (Keenan et al. 2004).
4.4.1.2 Endorphins
Fact sheet 4.14: Endorphins
Sequence:
YGGFMTSEKS QTPLVTLIKN AIIKNAYKKG
Synthesis and target:
In POMC expressing cells by the action of PC1
Function:
Endogenous opioid
Receptor:
Heptahelical G-protein-coupled receptor: 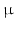 -opioid receptor (MOR)
-opioid receptor (MOR)
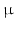 -opioid receptor (MOR)
-opioid receptor (MOR)Introduction
Endorphin s are endogenous opiates . These are released, for example, as a reaction to pain. Inasmuch as  -endorphin release is coupled to ACTH release, reactions to stress are metabolic changes, circulatory reactions mediated by glucocorticoids or adrenaline, and on the other hand analgesic effects by endorphins. Triggering the feeling of happiness by endurance sports appears to be an adaption to stress.
-endorphin release is coupled to ACTH release, reactions to stress are metabolic changes, circulatory reactions mediated by glucocorticoids or adrenaline, and on the other hand analgesic effects by endorphins. Triggering the feeling of happiness by endurance sports appears to be an adaption to stress.
 -endorphin release is coupled to ACTH release, reactions to stress are metabolic changes, circulatory reactions mediated by glucocorticoids or adrenaline, and on the other hand analgesic effects by endorphins. Triggering the feeling of happiness by endurance sports appears to be an adaption to stress.
-endorphin release is coupled to ACTH release, reactions to stress are metabolic changes, circulatory reactions mediated by glucocorticoids or adrenaline, and on the other hand analgesic effects by endorphins. Triggering the feeling of happiness by endurance sports appears to be an adaption to stress.Physiology
As shown in Fig. 4.19  -endorphin and ACTH are formed in equal amounts by the POMC processing by PC1. Endorphin synthesis takes place in all cells where MSH is formed. Endorphins thus can be released from all those cells/tissues where ACTH is formed and from those MSH forming cells in hypothalamus, pituitary, skin, additional tissues, and leukocytes.
-endorphin and ACTH are formed in equal amounts by the POMC processing by PC1. Endorphin synthesis takes place in all cells where MSH is formed. Endorphins thus can be released from all those cells/tissues where ACTH is formed and from those MSH forming cells in hypothalamus, pituitary, skin, additional tissues, and leukocytes.
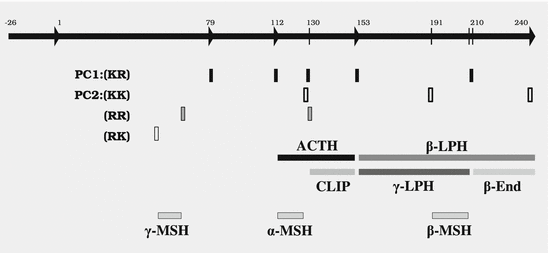
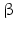 -endorphin and ACTH are formed in equal amounts by the POMC processing by PC1. Endorphin synthesis takes place in all cells where MSH is formed. Endorphins thus can be released from all those cells/tissues where ACTH is formed and from those MSH forming cells in hypothalamus, pituitary, skin, additional tissues, and leukocytes.
-endorphin and ACTH are formed in equal amounts by the POMC processing by PC1. Endorphin synthesis takes place in all cells where MSH is formed. Endorphins thus can be released from all those cells/tissues where ACTH is formed and from those MSH forming cells in hypothalamus, pituitary, skin, additional tissues, and leukocytes.
Fig. 4.19
POMC: prohormone convertases and alternative peptides. By the prohormone convertases PC1 and/or PC2, proopiomelanocortin (POMC) peptides are cleaved from the precursor: adrenocorticotropin (ACTH), lipocortin (LPH), melanocortin (MSH), endorphin (End), and corticotropin-like intermediate peptide (CLIP). PC1 cleaves but after Lys-Arg (KR), whereas PC2 cleaves after Lys-Lys (KK), Arg-Arg (RR), or Arg-Lys (RK). Which peptides are formed depends on the type of endocrine cell and of its convertase expression.
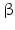 -Endorphin has a role in analgesia:
-Endorphin has a role in analgesia: 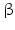 -endorphin is one endogenous ligand of
-endorphin is one endogenous ligand of 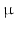 -opioid and
-opioid and 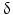 -opioid receptors (MOR and DOR). Both receptors are found in neurons that trigger pain, so-called nocireceptors communicating injury, chemical attack, heat, or coldness to the nocicenters of the brain. MOR neurons are densely found in the periaqueductal gray of the midbrain. As do other analgesics
-opioid receptors (MOR and DOR). Both receptors are found in neurons that trigger pain, so-called nocireceptors communicating injury, chemical attack, heat, or coldness to the nocicenters of the brain. MOR neurons are densely found in the periaqueductal gray of the midbrain. As do other analgesics 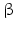 -endorphin blocks the activity of nocireceptors.
-endorphin blocks the activity of nocireceptors.It is not yet clear whether endorphins act directly or indirectly via other analgesic mediators. Endorphins 1 and 2 have, for example, a greater affinity to MOR than endorphins. However, the proteins where they should be cleaved from have not yet been found. Apart from endorphins and endomorphins, enkephalins and dynorphins are analgesically active as well.
Information about a phylogeny of endomorphins and nocireception has not yet been published.
4.4.1.3 Melanocortins
Fact sheet 4.15: 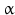 -,
-, 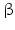 -, and
-, and 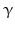 -Melanocyte stimulating hormone (MSH)
-Melanocyte stimulating hormone (MSH)
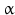 -,
-, 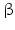 -, and
-, and 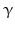 -Melanocyte stimulating hormone (MSH)
-Melanocyte stimulating hormone (MSH) Sequence:
 -MSH Acetyl-SYSMEHFRWGKPV-NH2
-MSH Acetyl-SYSMEHFRWGKPV-NH2




 -MSH Acetyl-SYSMEHFRWGKPV-NH2
-MSH Acetyl-SYSMEHFRWGKPV-NH2 Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree