Venous Thromboembolism
Venous thromboembolism (VTE) is a major health problem in the United States with more than 900,000 estimated cases annually.1 The average yearly incidence is 117 cases per 100,000 population, with higher rates in women of childbearing age, males over 45 years old, and the elderly (where rates are up to five-fold higher). Pulmonary embolism (PE) occurs about 60% as often as deep venous thrombosis (DVT) and has a high mortality; the incidence may be higher since the diagnosis is often missed in hospitalized patients.2,3 Chronic VTE-induced pulmonary hypertension is a late complication of PE in 1% to 4% of cases after 4 years of follow-up, with all cases occurring before 2 years.4,5 Most cases of DVT occur in the lower extremities, but virtually any venous vascular bed can be involved. Upper extremity DVTs represent 1% to 5% of the total and are usually associated with long-term indwelling central venous access devices, thrombophilia, and/or cancer.6 When upper extremity DVTs occur, the potential for subsequent pulmonary embolization is estimated to be as high as 36% in some trials,7 though fatal pulmonary embolism is less common than for DVT of the lower extremities.
![]()
DEEP VENOUS THROMBOSIS AND PULMONARY EMBOLISM
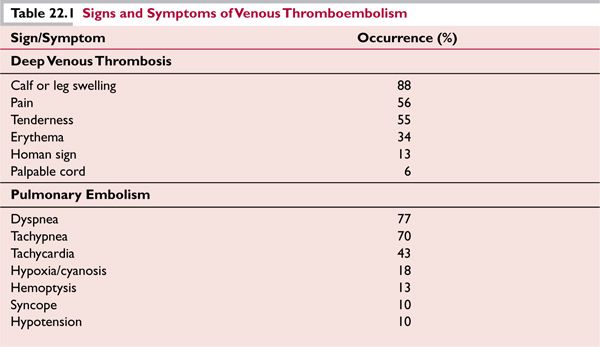
DVT of the extremity is often the precursor of PE, though both may become symptomatic at the same time, and it is possible that PE may occur without symptomatic DVT, due to complete embolism of nascent thrombus before complete occlusion of the vein occurs. One autopsy series indicates that a substantial number of patients with PE have no pathologic evidence for DVT.8 The signs and symptoms of DVT and PE are listed in Table 22.1.7,9
The postthrombotic syndrome (PTS) is an important complication of DVT. It is caused by venous hypertension from outflow obstruction and valvular injury and varies from mild edema with little discomfort to incapacitating limb swelling with pain and ulceration. The severity of PTS can be assessed using the Villalta scale,10 which is based on the cumulative rates of signs and symptoms characteristic of the syndrome (Table 22.2).
The reported incidence of PTS after an acute episode of DVT has been reported to range from 23% to 60%11; severe, disabling PTS with skin breakdown and ulceration is seen much less commonly, perhaps in <10% of cases, while mild symptoms probably are experienced in the majority of DVT cases. Surprisingly, contralateral extremities may also develop postthrombotic manifestations without prior evidence of overt DVT11; perhaps occult obstruction of the inferior vena cava (IVC) may be to blame. Sized-to-fit graded compression stockings (typically 20–40 mm pressure) should be applied shortly after diagnosis of DVT to prevent acute dilatation that may result in permanent damage to the valves. If there is poor circulation in the leg due to complete obstruction of venous outflow, compression stockings should be used cautiously or withheld if the increased compression threatens to stop blood flow altogether. After definitive therapy (thrombolysis or mechanical clot extraction) and venous flow is restored to some extent, compression stockings may be reconsidered to mitigate acute symptoms and eventual PTS. A sized-to-fit compression stocking can reduce the rate of PTS by about 50%.12 Compression stockings do not substitute for adequate anticoagulation but are useful adjuncts to exercise-based rehabilitation programs. Stockings that are not fitted to provide 20 to 40 mm Hg compression do not confer equivalent benefits as seen from fitted stockings. A poorly fitted stocking can actually be detrimental, for instance, when the upper parts of the stocking roll down and form a loose “tourniquet” and thereby impair return blood flow from the leg. Unfortunately, compression stockings or sleeves for upper extremity DVTs have not proven to be as beneficial, perhaps due to the lesser hydrostatic pressures involved with the upper extremity.
Distal DVT is defined as thrombus found below the popliteal vein trifurcation, and occurs most commonly (71% of the time) in the peroneal vein.13 The risk for PE is almost negligible, whether treated with anticoagulation, or not; propagation to other distal calf veins does occur in about half of all patients, and propagation to more proximal deep veins is seen in ~5% of patients.13,14 Lysis of isolated calf DVT typically occurs within 3 months.13 The risk of anticoagulation under these circumstances (0%–6% bleeding risk) is approximately equal to the benefits realized (less propagation), so it remains controversial whether anticoagulation is necessary. A recent systematic review suggests that anticoagulation or serial imaging with noninvasive methods may be equally valid strategies.15 PTS occurs in ~5% of patients in the long term but is not characterized by severe changes such as skin ulceration.15
Table 22.2 The Villalta Scale for Evaluation of Postthrombotic Syndrome (PTS)
Symptoms
Pain
Cramps
Heaviness
Paresthesia
Pruritus
Clinical Signs
Edema
Venous dilatation/ectasia
Hyperpigmentation
Erythema
Lipodermatosclerosis (skin induration)
Pain during calf compression
Characteristics of PTS. Scoring is based on the cumulative rating of the signs and symptoms, with each rated a 0 (absent), 1 (mild), 2 (moderate), 3 (severe).Total score: 0–4, no PTS; 5–14, mild to moderate PTS; ≥15 or presence of a venous ulcer, severe PTS.
![]()
IMAGING OF DEEP VENOUS THROMBOSIS
Venography remains the standard for diagnostic imaging of DVT, but it is used less commonly than ultrasonography since it is an invasive procedure that uses radiocontrast dye and requires a skilled operator to perform the injection. Further, there is the need to bring a patient to a fluoroscopy suite, which may not be feasible in the acutely ill patient in an intensive care unit with other comorbidities. In contrast, ultrasonography is noninvasive, portable, and does not use contrast to which the patient may be allergic. Doppler ultrasound instruments are typically portable and can be brought to the bedside even in the most acutely ill patients. Venography retains an advantage for diagnosis of small distal DVTs that are not well imaged by ultrasound, as well as thrombosis of the vena cava or iliac veins of the pelvis that are not accessible to ultrasound examination because they are obscured by bowel gas. Venography may also be useful in instances where ultrasound is not feasible or an unequivocal diagnosis of DVT must be made. Ultrasonography is considerably more sensitive for the detection of proximal DVT than for distal DVT.
Sensitivity of compression ultrasound with venous imaging ranges from 89% to 96% when DVT is diagnosed by a combination of direct visualization of an occlusive thrombus and noncompressibility of a vein. The specificity of this finding for DVT ranges from 94% to 99%, but, unfortunately, the sensitivity may be substantially diminished (47%–62%) in patients with asymptomatic DVT.16 Serial ultrasound testing (which has little risk to the patient in contrast to venography) improves sensitivity, as a previously undiagnosed distal DVT may declare itself by proximal propagation. Furthermore, an ultrasound can accurately diagnose certain conditions that occasionally mimic DVT, such as Baker cyst. Impedance plethysmography may be useful in differentiating between a new or recurrent DVT, especially if the previous DVT has not resolved.
Magnetic resonance venography (MRV) and computed tomography venography (CTV) can diagnose DVT in a noninvasive manner. Prospective studies comparing CTV with venous ultrasound for diagnosis of DVT reported sensitivity rates of 100% and specificity of 96% to 100%.17,18 CTV can be easily combined with computed tomography angiography in patients suspected to have PE. However, it always requires the administration of IV contrast. One prospective blinded study reported the sensitivity to be >94% and the specificity to be >90% for the diagnosis of DVT using noncontrast MRV direct thrombus imaging.19 A major advantage for CTV and MRV is that deep abdominal, pelvic, and calf veins can be imaged. Furthermore, MRV can be successfully used avoiding contrast and its risks (such as gadolinium-associated systemic fibrosis in patients with chronic kidney disease20,21). Major disadvantages include cost, availability, expert reading, and possible need for IV contrast use when compared to ultrasound techniques.
![]()
PULMONARY EMBOLISM DIAGNOSIS: ECHOCARDIOGRAPHY, ELECTROCARDIOGRAPHY, AND X-RAY
PE is thought to be the consequence of clot breaking free from a lower extremity DVT. Upper extremity DVT embolization is less common, but the increasing use of central venous access devices for cancer chemotherapy or other long-term parenteral treatment may increase its frequency. Less commonly, PE may originate in the IVC (particularly in association with renal cell carcinoma) or the right ventricle of the heart from mural thrombus. There is intriguing evidence from the study of acute trauma patients8 that pulmonary “embolism” can be seen without concurrent DVT, raising the possibility that some pulmonary emboli may actually be in situ pulmonary thrombosis. Regardless of the mechanism, imaging approaches to diagnose pulmonary embolism include pulmonary angiography (the standard), CT angiography, ventilation/perfusion scanning, and more recently MRI. Echocardiographic confirmation of right ventricular hypertension may assist in the decision regarding thrombolysis with right heart failure. Both echocardiography and spiral CT have a low sensitivity for PE located in peripheral pulmonary vessels. In severe cases chest x-ray findings may include a Hampton hump (a wedge-shaped opacity with apex pointing to the hilum) or a focal paucity of blood vessel perfusion. Ancillary diagnostic chest radiography, ECG, and echocardiographic findings suggestive of PE are listed in Table 22.3.
![]()
LABORATORY DIAGNOSIS OF VENOUS THROMBOEMBOLISM
The D-dimer is a quantitative assay with good reproducibility that is often automated and can be used as an adjunct to imaging studies to exclude the presence of PE or DVT.22–24 When a thrombus is degraded by plasmin, D-dimers and other fibrin split products are formed from cross-linked chains of the fibrin clot—as such, they are markers for fibrin turnover. The use of D-dimer is preferable to the semi-quantitative fibrin split products that are measured by latex agglutination methods at varying dilutions of patient plasma. If a patient has a low pretest probability of VTE and the sensitive ELISA test for D-dimers is negative, as a practical matter VTE can be excluded. The low specificity for VTE requires that further diagnostic testing be conducted in the event of an elevated D-dimer to elucidate its etiology. Many other conditions can cause elevation of the D-dimer, including cancer, pregnancy, sepsis, sickle cell crisis, acute myocardial infarction, cardiopulmonary resuscitation, excessive bleeding, trauma, and recent surgery. Measurement of D-dimers may also guide the duration of anticoagulation for VTE, since the optimal course of therapy for such patients has not been established.25
The PROLONG Study indicated that patients with positive D-dimers 1 month after the completion of at least 3 months anticoagulation with vitamin K antagonists (VKAs) for idiopathic VTE had a significantly higher risk for recurrent VTE, which was mitigated by prolonged anticoagulation. Patients with abnormal D-dimers who did not resume anticoagulation experienced a 15% incidence of rethrombosis over the 18-month observation period compared to 2.9% if anticoagulation was restarted.26 The adjusted hazard comparing the rates of recurrence was 4.26 (95% CI 1.23–14.6, p = 0.02). A follow-up study, the PROLONG II, assessed the utility of repeated D-dimer testing in patients with a first unprovoked episode of VTE with normal D-dimer 1 month after stopping VKAs. D-dimer was tested at study initiation and every 2 months thereafter with a follow-up period of 13 months; 14% of patients with an initially negative D-dimer had a positive test at month 3 of evaluation. Furthermore, the D-dimer became abnormal at each subsequent time point in about 10–15% of patients up to 9 months of follow-up, at which time it decreased to 8–10%. An abnormal D-dimer at the first measurement or at day 30 usually remained abnormal over time in the majority of cases, and this pattern was associated with an increased risk of rethrombosis. The rates of recurrence for patients with an abnormal D-dimer at 3 months was 22.6% (95% CI 10%–41%) compared to 4.6% (95% CI 2%–9%) if D-dimer was normal (p = 0.003).27 Repeat D-dimer testing may help identify the subgroup of patients at lower risk for recurrence, in whom anticoagulation could be stopped. A multicenter prospective study (DULCIS, http://clinicaltrials.gov:NCT00954395) is currently underway to clarify this issue.
Table 22.3 Ancillary Findings Diagnostic for Pulmonary Embolism
Chest Radiography
Hampton hump
Focal paucity of blood vessel perfusion (Westermark sign)
Dilated pulmonary artery proximal to the thrombus
Atelectasis
Pleural effusion
Elevated diaphragm
Electrocardiography
New right bundle branch block
S1Q3T3 pattern (sign of acute cor pulmonale)
Supraventricular arrhythmias
Echocardiography
Right ventricular dilatation, often with myocardial hypokinesis
Pulmonary artery dilatation
Right ventricular mural thrombi
Tricuspid regurgitation
Loss of inspiratory collapse of the inferior vena cava
![]()
DEEP VENOUS THROMBOSIS IN SITES OTHER THAN THE DISTAL VEINS OF THE LOWER EXTREMITIES
Aside from typical presentations involving the lower extremities, DVT can occur in other sites such as veins of the upper extremities (particularly in conjunction with central venous catheters commonly used in cancer patients for chemotherapy), or in the veins of the chest or abdomen. Bilateral upper extremity DVTs are uncommon and should prompt a search for malignancy. The effects of DVT in these sites can be devastating, even in the absence of PE. Perioperative DVT of the splanchnic veins is common, occurring more frequently in laparoscopic procedures than with open ones. Unprovoked DVT of the splanchnic vessels should prompt a search for underlying abnormalities of hemostasis, such as deficiency of anticoagulant proteins, undiagnosed cancer, hematologic diseases such as myeloproliferative disorders (MPD), or paroxysmal nocturnal hemoglobinuria (PNH).
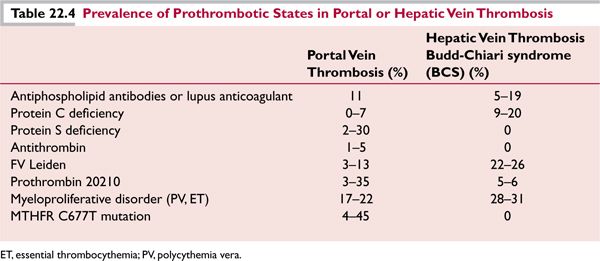
Portal vein thrombosis can commonly occur as a complication of surgical procedures, especially splenectomy, or during pregnancy, or with peritonitis. In cases that are not associated with a precipitating factor, antiphospholipid antibodies, deficiency of protein C, protein S, or (less commonly) anti-thrombin III may be found; the factor V Leiden and prothrombin 20210 gene polymorphisms may also be seen in patients at rates greater than in the general population (as is true in any group of patients with pathologic thrombosis). Clonal V617F point mutation in the Janus 2 kinase (JAK2V617F) tyrosine kinase gene occurs in a large proportion of MPD (particularly polycythemia rubra vera), and is found in 45% of BCS and 34% of portal vein thrombosis. Table 22.4 indicates the prevalence of various prothrombotic states in patients with portal or hepatic vein thrombosis.28–30
Treatment options for splanchnic vein thrombosis include anticoagulation, thrombolysis, and in the most extreme cases consideration should be given to orthotopic liver transplantation for those with liver failure. In addition, acute reduction of red blood cell mass and/or platelet count may be beneficial if the splanchnic vein thrombosis is due to polycythemia vera (PV) or essential thrombocythemia.
![]()
ACQUIRED THROMBOPHILIC STATES
Heparin-Induced Thrombocytopenia and Heparin-Induced Thrombocytopenia with Thrombosis
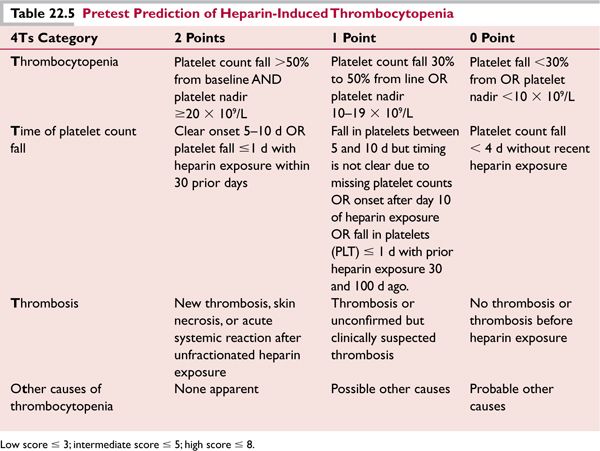
Heparin-induced thrombocytopenia (HIT) is a prothrombotic state caused by a drug reaction to unfractionated heparin (UFH) and less commonly to low molecular-weight heparin (LMWH), which can also exacerbate HIT due to cross reactivity with UFH. Even small exposures to UFH, including flushing of intravenous lines, may precipitate HIT and even thrombosis, in patients with antibodies. Antibodies of the IgG class are formed that produce a strong activation of platelets through their FcγIIa receptors. They recognize large multimolecular complexes of platelet factor 4 bound to heparin (PF4/H), although only about 10% of all anti-PF4/H antibodies have platelet-activating properties.31 This activation promotes thrombosis in vivo in both venous and arterial sites. HIT is a “clinical-pathologic syndrome,” requiring both a compatible clinical picture and positive laboratory test results. In general, platelet counts begin to decrease 5 to 9 days after the initiation of heparin. Thrombocytopenia and thrombosis may occur earlier in patients primed by heparin administration in the prior 100 days. Clinical scoring systems have been implemented to evaluate the pretest probability of HIT: 4Ts32 and most recently the HIT Expert Probability (HEP) score33 (Tables 22.5 and 22.6).
Low scores usually indicate a <2% probability of having a positive platelet activation assay. Two types of assays are used to establish diagnosis: enzyme immunoassays, such as an ELISA that detects PF4-heparin antibodies, and platelet activation/aggregation assays which detect spontaneous aggregation of platelets induced by the addition of heparin to the patient’s platelet rich plasma. Sensitivity of platelet aggregation assays can be increased when patient’s platelets are “loaded” with radioactive serotonin; serotonin release from platelets is detected as a marker of platelet activation in vitro, after the addition of heparin. The most sensitive test is the serotonin-loaded platelet aggregation assay. False positive results are common with the ELISA test, and its specificity varies depending on the pretest clinical probability. A negative result usually excludes HIT.
Two different classes of anticoagulants are used for the treatment of HIT in the presence or absence of thrombosis: direct thrombin inhibitors, lepirudin, bivalirudin, and argatroban, which are approved in the United States for the treatment of HIT and the factor Xa inhibitor fondaparinux, which is currently approved for DVT prophylaxis and treatment, but has growing evidence in literature to be an effective treatment for HIT.34 Fondaparinux binds anti-PF4 antibodies but does not activate platelets, unlike traditional LMWH preparations such as enoxaparin, dalteparin, and tinzaparin, which crossreact with the PF4-heparin antibodies and can activate platelets. Fondaparinux binds irreversibly to factor Xa and has a long active half-life (17 hours), which may prevent rebound hypercoagulability, but cannot be readily reversed if bleeding ensues. Dosing needs to be adjusted for impaired renal function. Lepirudin and argatroban require intravenous continuous infusions due to their short half-lives (<2 hours), and need PTT monitoring. Lupus anticoagulants (LAC) and/or high factor VIII levels may result in prolonged or shortened clotting times, respectively, thereby complicating monitoring. Algorithms exist for infusions of argatroban without monitoring when LAC interfere with monitoring.35 Levels of argatroban or other direct thrombin inhibitors can be monitored more directly by use of assays of the ecarin clotting time; ecarin is a snake venom that converts fibrinogen to fibrin but is not influenced by LAC or levels of factor VIII.36 Lepirudin is antigenic, and antibody formation results in excessive anticoagulation. Lepirudin is contraindicated in the presence of renal insufficiency. Bivalirudin (approved in HIT specifically for patients undergoing percutaneous coronary intervention) and argatroban are both hepatic-excreted, nonantigenic, and short-acting, and they should be used cautiously in patients with liver disease. Initiation of warfarin should be postponed at least until the platelet count is above 150,000/μL, since warfarin use during acute HIT is a major risk factor for venous limb gangrene, perhaps on the basis of rapid depletion of protein C, a vitamin-dependent anticoagulant protein with a short half-life (6–7 hours).
Nephrotic Syndrome
Patients with nephrotic syndrome are at higher risk for VTE, arterial thromboembolism (ATE), and renal vein thrombosis than is the general population [annual incidence, 9.85% (VTE) and 5.52% (ATE)].37 Membranous glomerulonephritis confers the highest risk of VTE. Hypercoagulability appears due to alterations in plasma levels of proteins involved in coagulation and fibrinolysis. The anticoagulant proteins antithrombin III and protein S are lost in the urine, perhaps due to their relatively small size compared to procoagulant factors V, VIII, von Willebrand factor, and fibrinogen, which may be retained by the kidney.38 Further, fibrinogen, factor VIII, and von Willebrand factor are acute-phase reactants, and their levels may be increased by inflammation.39 The routine use of prophylactic anticoagulation for patients with nephrotic syndrome has not been established by randomized controlled trials, but some experts advocate this when additional risk factors such as membranous glomerulonephritis or the antiphospholipid syndrome are present. LMWH must be used with extreme caution in patients with renal insufficiency in addition to nephrotic syndrome.
Antiphospholipid Antibody Syndrome
Antiphospholipid antibodies (APA) may be associated with thrombosis on the basis of various mechanisms,40 summarized in Table 22.7. Anticardiolipin antibodies are APA associated with infections such as syphilis, but these are transient and not usually associated with thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree