Chapter 19
Vector-Borne Diseases
At the conclusion of this chapter, the reader should be able to:
• Describe the etiology, epidemiology, and signs and symptoms of Lyme disease.
• Analyze the immunologic manifestations and diagnostic evaluation of Lyme disease.
• Explain the principle, interpretation, and limitations of an antibody detection assay.
• Describe prevention strategies of Lyme disease.
• Summarize the etiology, epidemiology, and signs and symptoms of ehrlichiosis.
• Analyze the immunologic manifestations and diagnostic evaluation of ehrlichiosis.
• Explain the prevention of ehrlichiosis.
• Summarize the etiology, epidemiology, and signs and symptoms of Rocky Mountain spotted fever.
• Analyze the immunologic manifestations and diagnostic evaluation of Rocky Mountain spotted fever.
• Explain the prevention of Rocky Mountain spotted fever.
• Summarize the etiology, epidemiology, and signs and symptoms of babesiosis.
• Analyze the immunologic manifestations and diagnostic evaluation of babesiosis.
• Explain the prevention of babesiosis.
• Briefly discuss the etiology and laboratory diagnosis of West Nile virus infection.
• Analyze case studies related to the immune response in Lyme disease, Ehrlichiosis, and Babesiosis.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle, limitations, and clinical applications of the rapid Borrelia burgdorferi antibody detection assay.
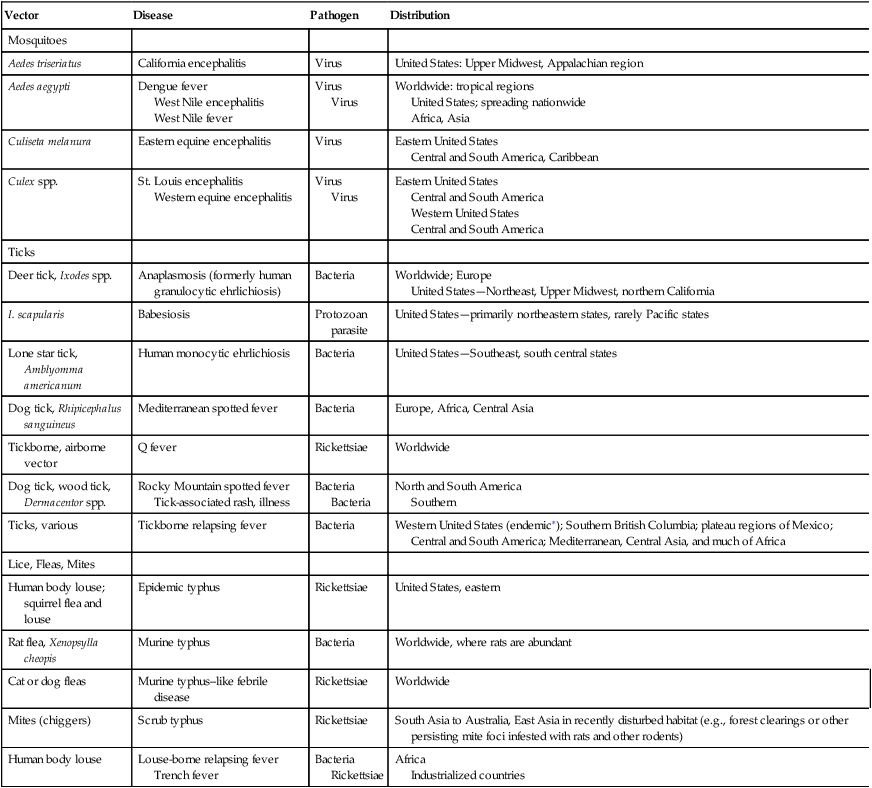
Globalization has made the world a more connected place. Bacterial and viral diseases transmitted by mosquitoes, ticks, and fleas continue to be an ever-present threat worldwide (Table 19-1). Some of these diseases have been present in the United States for a long time but others have emerged recently. These include some of the world’s most destructive diseases, many of which are increasing threats to human health as the environment changes and globalization increases.
Table 19-1
Examples of Vector-Borne Diseases
| Vector | Disease | Pathogen | Distribution |
| Mosquitoes | |||
| Aedes triseriatus | California encephalitis | Virus | United States: Upper Midwest, Appalachian region |
| Aedes aegypti | Dengue fever West Nile encephalitis West Nile fever | Virus Virus | Worldwide: tropical regions United States; spreading nationwide Africa, Asia |
| Culiseta melanura | Eastern equine encephalitis | Virus | Eastern United States Central and South America, Caribbean |
| Culex spp. | St. Louis encephalitis Western equine encephalitis | Virus Virus | Eastern United States Central and South America Western United States Central and South America |
| Ticks | |||
| Deer tick, Ixodes spp. | Anaplasmosis (formerly human granulocytic ehrlichiosis) | Bacteria | Worldwide; Europe United States—Northeast, Upper Midwest, northern California |
| I. scapularis | Babesiosis | Protozoan parasite | United States—primarily northeastern states, rarely Pacific states |
| Lone star tick, Amblyomma americanum | Human monocytic ehrlichiosis | Bacteria | United States—Southeast, south central states |
| Dog tick, Rhipicephalus sanguineus | Mediterranean spotted fever | Bacteria | Europe, Africa, Central Asia |
| Tickborne, airborne vector | Q fever | Rickettsiae | Worldwide |
| Dog tick, wood tick, Dermacentor spp. | Rocky Mountain spotted fever Tick-associated rash, illness | Bacteria Bacteria | North and South America Southern |
| Ticks, various | Tickborne relapsing fever | Bacteria | Western United States (endemic∗); Southern British Columbia; plateau regions of Mexico; Central and South America; Mediterranean, Central Asia, and much of Africa |
| Lice, Fleas, Mites | |||
| Human body louse; squirrel flea and louse | Epidemic typhus | Rickettsiae | United States, eastern |
| Rat flea, Xenopsylla cheopis | Murine typhus | Bacteria | Worldwide, where rats are abundant |
| Cat or dog fleas | Murine typhus–like febrile disease | Rickettsiae | Worldwide |
| Mites (chiggers) | Scrub typhus | Rickettsiae | South Asia to Australia, East Asia in recently disturbed habitat (e.g., forest clearings or other persisting mite foci infested with rats and other rodents) |
| Human body louse | Louse-borne relapsing fever Trench fever | Bacteria Rickettsiae | Africa Industrialized countries |
∗Most recent cases and outbreaks have occurred in rustic cabins at higher elevations (≥8000 ft) in coniferous forests in the western United States.
Some of the newly emerging infectious diseases in the United States include the following:
Lyme Disease
Etiology
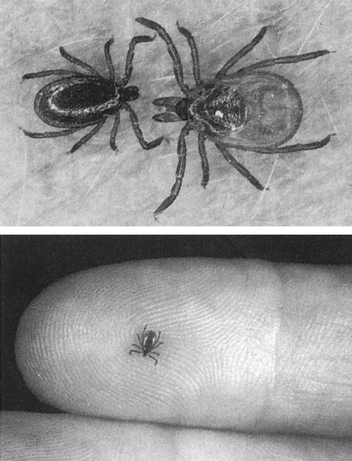
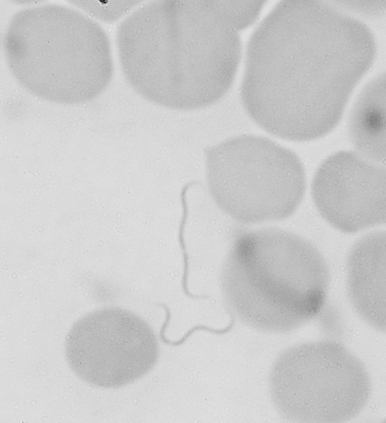
Lyme disease (Lyme borreliosis) is caused by a spirochete bacterium. It is a cutaneous systemic infection generally transmitted by a hard-bodied tick (Fig. 19-1) and caused by Borrelia burgdorferi (Fig. 19-2). The causative agent of Lyme borreliosis currently consists of three pathogenic species—B. burgdorferi, Borrelia afzelii, and Borrelia garinii. Only B. burgdorferi strains have been found in the United States. In contrast, most of the illness in Europe is caused by B. afzelii, which is associated with the chronic skin condition acrodermatitis chronica atrophicans (ACA), and B. garinii, which is associated with neurologic symptoms. Only these two species have been found in Asia. The complete genome of B. burgdorferi (strain B31) has now been sequenced.
Epidemiology
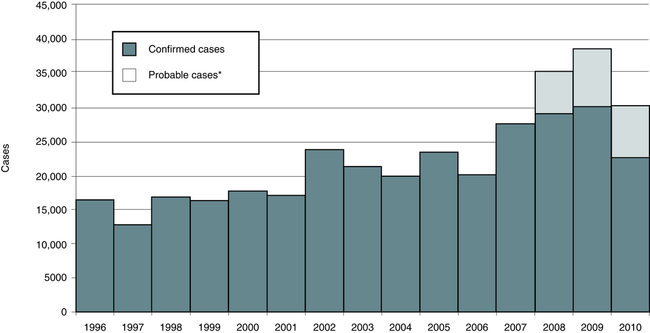
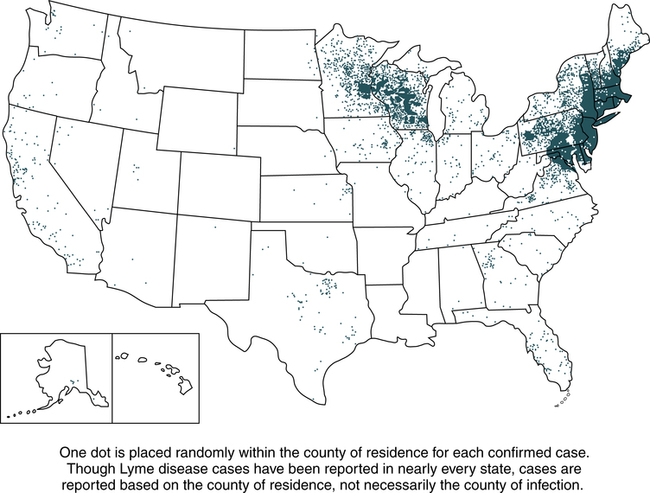
Lyme disease does not occur nationwide and is concentrated heavily in the northeast and upper midwest. The highest number of confirmed cases of Lyme disease to date was 29,959 in 2009 (Fig. 19-3). Persons of all ages and both genders are equally susceptible. In 2011, 96% of Lyme disease cases were reported from 13 states (Fig. 19-4):
Signs and Symptoms
Lyme borreliosis is a multisystem illness that primarily involves the skin, nervous system, heart, and joints (Table 19-2). Lyme disease usually begins during the summer months with EM and flulike symptoms and may be accompanied by right upper quadrant tenderness and a mild hepatitis (stage 1). This stage is followed weeks to months later by acute cardiac or neurologic disease in a minority of untreated individuals (stage 2) and then by arthritis and chronic neurologic disease (stage 3) in many untreated patients weeks to years after disease onset. There is considerable overlap of these stages, but Lyme disease is best characterized as an illness that evolves from early to late disease without reference to an arbitrary staging system. However, a patient may have one or all of the stages, and the infection may not become symptomatic until stage 2 or 3. Most affected patients have EM and 25% manifest arthritis; neurologic manifestations and cardiac involvement are uncommon.
Table 19-2
Clinical Features of Lyme Disease
| Stage | Duration | Signs and Symptoms |
| I | 4 wk (median) after injection | Cutaneous manifestations (erythema migrans) or other skin eruptions, flulike syndrome, neurologic symptoms |
| II | Follows a variable latent period | Target organs and systems include nervous system, heart, eyes, and skin, all of which can manifest abnormalities |
| III | Weeks to years after infection | Arthritis, late neurologic complications, acrodermatitis chronica atrophicans |
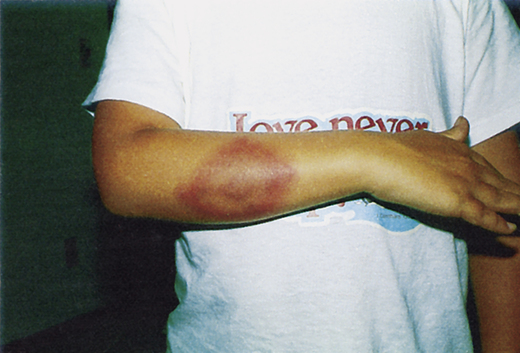
Cutaneous Manifestations
Cutaneous manifestations can be demonstrated as early ECM (Fig. 19-5), secondary lesions (disseminated lesions and lymphocytoma), and late lesions (ACA). Except for the late lesions, cutaneous manifestations generally resolve spontaneously over weeks to months. The red papule at the site of the tick bite is most often located on the thigh, groin, or axilla. Facial EM is seen more frequently in children.