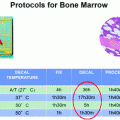
Fig. 1
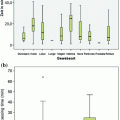
Validation trial #1 main hospital specimens
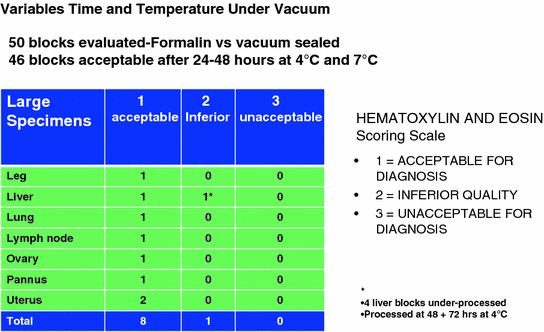
Fig. 2
TissueSAFE histologic assessment of morphologic preservation
2 Validation Trial #2—Defining the Transport System from Community Hospital
One of our chief goals was to transport human tissues in the fresh state from a community hospital 25 miles away to a continuous flow core laboratory for dissection and processing (Fig. 3). Because we had to satisfy the requirement of five courier runs per day from the community hospital to the core laboratory, we innovated a means of transporting the vacuum sealed specimens in an insulated cooler between layers of plastic grids with conventional ice cubes in Ziploc bags place above and below the specimens (Fig. 4). An RFID card was included with each specimen run to record a temperature and time log for the transport run (Fig. 5). The optimal temperature achieved for transport with this mechanism was a stable 4 °C. In this pilot we evaluated 11 medium and large size tissue specimens assessed at transport delay times of 24 and 48 h on ice at 4 °C. All were judged to be acceptable for diagnosis by pathologists. The histologic assessment showed no differences in quality for specimens held in the chilled, airless state for 24 and 48 h (Fig. 6).
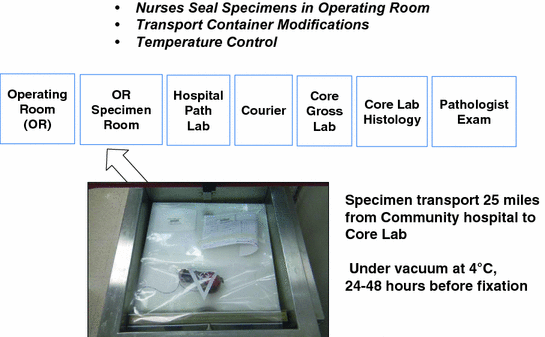

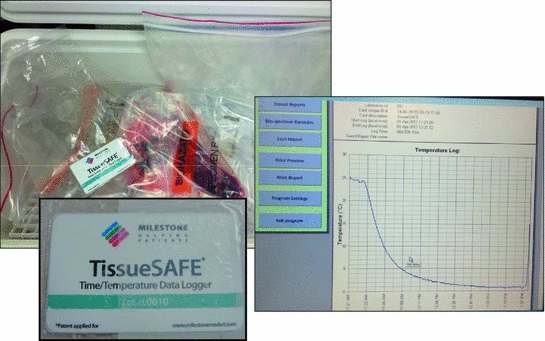
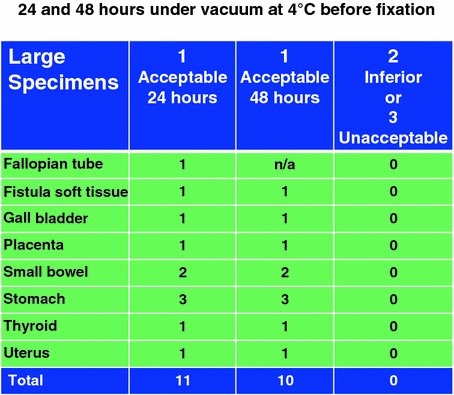

Fig. 3
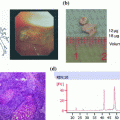
Validation trial #2 transport system from community hospital

Fig. 4
Transport by insulated cooler with conventional ice cubes in Ziploc bags

Fig. 5
RFID card records the run temperature

Fig. 6
TissueSAFE histologic assessment of morphologic preservation after transport in coolers on ice at 4 °C
The TissueSAFE device has been subsequently located in the OR area and OR circulator nurses have been trained to triage and seal the specimens. We have now transported hundreds of specimens (roughly 56 % of OR based surgical volumes) from this community hospital in this manner for the past year without incident or pathologist complaint. The process receives very high marks from OR leadership and nurses. Large cubes of formalin are no longer stored and used in this OR suite to fill large specimen containers. Only small biopsy containers prefilled with small amounts of formalin are used in these ORs for small specimens and needle biopsies. The enhanced safety from reduced exposure of OR personnel to formalin is considered priceless by OR leadership.
Based on this TissueSAFE process, this community hospital laboratory has used 135 fewer gallons of formalin for an annual cost savings of $1,688. The cost of consumables, plastic sealing bags replacing plastic bucket, was neutral. Additional savings not calculated are the courier fuel costs of transporting heavier specimen containers that would have been filled with formalin.
3 Validation Trial #3—Evaluating the Histology of Wider Variety of Large Specimens
In this trial we obtained a wider variety of tissues from the ORs of the Main Hospital for histologic evaluation after vacuum sealing with the TissueSAFE device located in our Pathology specimen receipt adjacent to the large theater of 32 ORs. These sealed specimens were then transported at intervals throughout the day at 4 °C to the core gross lab within another building at transport times of 1–10 h.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree