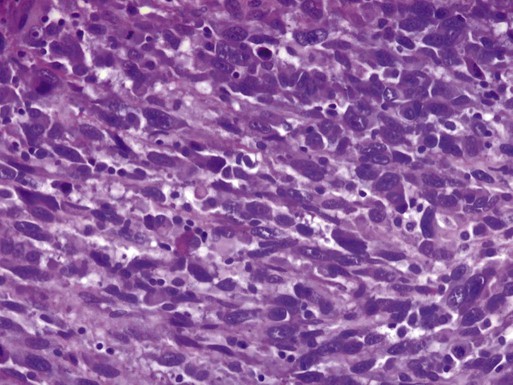
John F. Boggess and Joshua E. Kilgore • 46,000 estimated new cases per year • Approximately 8,100 deaths per year from the disease • Endometrioid adenocarcinoma arises in the endometrium and accounts for approximately 90% of cases • Most common presenting symptom is irregular vaginal bleeding • Risk factors include unopposed estrogen therapy, obesity, chronic anovulation, tamoxifen use, diabetes, and nulliparity • Staging is surgical according to International Federation of Gynecology and Obstetrics (FIGO) guidelines • Uterine sarcomas and carcinomas are staged differently • Primary surgery with hysterectomy, bilateral salpingo-oophorectomy and lymphadenectomy is curative in majority of cases • Radiation alone may be used for inoperable patients and/or palliation • Risk factors for recurrence or extrauterine spread include deep myometrial invasion, moderate- to high-grade histology, and lymphovascular space invasion (LVSI) • Adjuvant radiation may be used in patients with high-intermediate and high-risk disease • Adjuvant chemotherapy with or without radiation therapy may be administered in cases with aggressive histology, such as serous, clear cell, carcinosarcoma, or leiomyosarcoma and undifferentiated endometrial sarcoma Locally Advanced, Metastatic, or Recurrent Disease • Chemotherapy, radiation, or combination therapy is used in locally advanced, metastatic, or recurrent endometrial cancer • Cytoreductive surgery is associated with improved overall survival • Salvage radiotherapy is utilized for recurrences after surgery alone • Endocrine therapy with progestins and/or tamoxifen may be used when avoidance of cytotoxic chemotherapy is desired • Tumor-directed radiation therapy may be employed in cases of locally advanced, metastatic, or recurrent disease • Survival is strongly correlated with surgical stage, tumor grade, and histology • Uterine clear cell and serous carcinomas are associated with a worse prognosis than endometrioid adenocarcinomas Endometrial cancer, or uterine cancer, is a malignancy arising from the endometrium. Women have a 1-in-40 lifetime risk of being diagnosed with endometrial cancer, the fourth most common malignancy among women. Uterine cancer is the most common gynecologic malignancy in the United States.1 Stage I malignancies comprise the majority of endometrial cancers. Postmenopausal bleeding is the most common presentation. Cancer arising from endometrial glands is referred to as carcinoma, compared to the less common uterine sarcoma that arises in mesenchymal elements such as smooth muscle or connective tissue. An estimated 46,000 new diagnoses of uterine cancer and 8,100 deaths from the disease occurred in 2011.2 Median age at diagnosis is 63 years. The Surveillance, Epidemiology, and End Results program (SEER) reported that 70% of patients are diagnosed with localized disease, 17% with regional spread, 9% with distant disease, and 4% were unstaged.1 Five-year survival rates are approximately 80% to 90% for disease confined to the uterus (Table 88-1).3 Table 88-1 Endometrial cancer is uncommon in premenopausal women and genetic factors account for only 1% of newly diagnosed cases.4,5 Lynch syndrome or hereditary nonpolyposis colorectal cancer syndrome (HNPCC) is associated with a relative risk of 1.5 for the development of endometrial cancer before menopause.5 Women with Lynch syndrome have a 27% to 71% risk of endometrial cancer.6,7 Mutations in the DNA mismatch repair genes impair the ability to maintain genomic integrity. Patients with Lynch syndrome have a germline mutation in one mismatch repair allele and the second allele is inactivated through mutation, loss of heterozygosity, or epigenetic silencing by promoter hypermethylation. Inactivation of both genes leads to increased DNA mutations and alteration of microsatellite regions in the tumor compared to normal tissue. Microsatellite instability refers to the contraction or expansion of short repetitive DNA sequences and is due to loss of DNA mismatch repair. Microsatellite instability is found in 90% of tumor tissue from patients with Lynch syndrome. Mutations in MSH2 or MLH1 account for approximately 90% of the identified mutations associated with Lynch syndrome. Other mutations in PMS1, MSH6, and MLH3 have also been described. The age of diagnosis in women with Lynch syndrome is 46 to 54 years.8–11 Endometrioid histology is the most common Lynch syndrome–associated endometrial cancer; however, nonendometrioid tumors have been reported.12,13 The majority of tumors are diagnosed with early-stage disease, similar to women with sporadic endometrial cancer. Women with Lynch syndrome are at risk for synchronous or metachronous cancers.14,15 The Amsterdam Criteria have been developed to identify families at risk for Lynch syndrome (Table 88-2). The Society of Gynecologic Oncologists published committee guidelines for genetic testing of individuals at risk for Lynch syndrome (Table 88-3).16 Table 88-2 Table 88-3 Society of Gynecologic Oncologists Statement Guidelines on Risk Assessment for Lynch Syndrome 1. Patients with endometrial or colorectal cancer before age 50 years 2. Patients with endometrial and/or ovarian cancer and another Lynch-associated malignancy before age 50 years 3. Patients with endometrial or colorectal cancer and a first-degree relative with a Lynch syndrome–associated malignancy before age 50 year 4. Patients with endometrial or colorectal cancer at any age with 2 or more first- or second-degree relatives of any age with a Lynch syndrome–associated malignancy 5. Patients with first- or second-degree relative who meets above criteria From Lancaster JM, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 2007;107(2):159–62. Table 88-4 lists many of the known risk factors for endometrial adenocarcinomas. Endogenous estrogen exposure associated with nulliparity, early menarche, late menopause, obesity, and estrogen-producing tumors are associated with an increased risk of endometrial cancer.17,18 Risk factors are associated with the unopposed estrogen effect, or lifetime estrogen exposure. Exposure to unopposed estrogen leads to increased endometrial cell proliferation, resulting in increased DNA replication errors and somatic mutations. Exogenous estrogen sources, such as hormone replacement therapy without progestins, increases the risk for endometrial cancer fivefold.19 The use of progestins with hormone replacement therapy may decrease the risk of uterine cancer through downregulation of hormone receptors. Cancers that occur in women on combined hormone replacement therapy tend to be of low stage and grade. Endogenous hormones, such as androstenedione, estrone, and estradiol, are associated with a threefold to fourfold increased risk.20 Table 88-4 Risk Factors for Endometrial Cancer The National Surgical Adjuvant Breast Project (NSABP) and the Netherlands Cancer Institute clarified a causal link between the treatment of breast cancer with tamoxifen and endometrial cancer.21,22 Tamoxifen, a selective estrogen receptor modulator, was first described as a risk factor for uterine cancer in 1985. The NSABP evaluated 2834 women treated for breast cancer treated with tamoxifen. Twenty-four cases of endometrial cancer were diagnosed among the study patients. Nearly 96% (N = 23) of endometrial cancer cases occurred in women taking tamoxifen. The annual hazard rate of endometrial cancer development was 1.6 per 1000 patient-years in the tamoxifen group and 0.2 per 1000 patient-years in the placebo group. In addition, 88% of endometrial cancers diagnosed were stage I, and 78% were of low or intermediate grade. The use of tamoxifen results in a 38% improvement in disease-free survival for breast cancer, which far outweighs the risk of endometrial cancer, from which there were only four deaths. A subsequent analysis by the NSABP showed the risk was predominately in women 50 years of age or older, with an incidence of approximately 2 per 1000.23 Regular gynecologic follow-up is recommended for patients on tamoxifen. The Netherlands Cancer Institute performed a case–control study by identifying 98 patients in whom endometrial cancer developed after treatment for breast cancer compared with controls in whom endometrial cancer did not develop.24 Twenty-four percent of the patients with endometrial cancer had taken tamoxifen, compared with 20% of the controls. Treatment with tamoxifen for 5 years or more was associated with a 3.0 risk of endometrial cancer compared with controls. The etiology of uterine sarcomas is not well understood, but may be related to prior exposure to pelvic irradiation. Uterine sarcomas and carcinomas have been reported after irradiation for cervix and rectal cancer.25,26 Radiation-associated uterine cancers are usually of higher grade and stage, with a more unfavorable histology.27 Systemic disease and lifestyle factors influence the risk of endometrial cancer. Hypertension, diabetes, and obesity all increase the risk. Nearly 40% of uterine cancers can be attributed to obesity.28 Increasing body mass indices (BMIs) are associated with higher relative risks of developing a uterine malignancy. Overweight women (BMI 28 to 29.9) have a relative risk of 1.5 compared with women with a normal BMI. Obese women (BMI 30 to 33.9) have a relative risk of 2.9 and markedly obese women (BMI >34) have a relative risk of 6.3.29 The increased risk of endometrial carcinoma associated with increasing BMI may be explained by higher levels of endogenous estrogen. The conversion of androstenedione to estrone and the aromatization of androgens to estradiol occurs in peripheral adipose tissue. Severely obese women are more likely than nonobese women to have a less-aggressive histology and present with stage I disease.30 Known protective factors against endometrial cancer include full-term pregnancy, multiparity, older age of menarche, and oral contraceptive use. Use of oral contraceptives for up to 5 years is associated with a relative risk of 0.2, and its use for at least 1 year reduces endometrial cancer risk by approximately 45%.31 Interestingly, cigarette smoking decreases the risk of endometrial cancer; however, the clear adverse risks of smoking greatly outweigh any potential benefit with respect to endometrial cancer. Abnormal uterine bleeding is the most common presentation in women diagnosed with endometrial carcinoma. Approximately 80% of women with endometrial carcinoma present with abnormal uterine bleeding; however, the amount of bleeding does not correlate with the risk of cancer.32,33 Less commonly, abnormal cervical cytology may be the first indication of uterine cancer. The risk of endometrial carcinoma and the need for endometrial evaluation depends on age, symptoms, and the presence of risk factors.32,34–36 Postmenopausal bleeding, including spotting, carries a 3% to 20% risk of endometrial carcinoma. Diagnosis of endometrial carcinoma before age 45 years is uncommon; however, intermenstrual bleeding or prolonged periods of amenorrhea (6 or more months) after age 45 years should be evaluated. Nineteen percent of endometrial carcinomas occur between ages 45 and 64 years.37 The risk of uterine cancer before age 45 years is low and increases with advancing age. The majority of abnormal bleeding is due to benign uterine pathology, but further evaluation is warranted and recommended by the American College of Obstetricians and Gynecologists.36 Women presenting with clinical signs suspicious for endometrial carcinoma should undergo a physical examination, including pelvic examination. Urine or serum human chorionic gonadotropin testing to exclude pregnancy should be performed on all reproductive-age women prior to any endometrial sampling. Pelvic ultrasound is often ordered to assess the endometrial thickness. In postmenopausal women, transvaginal ultrasound to evaluate the endometrial thickness may be used for endometrial neoplasia in selected women. In women with postmenopausal bleeding, an endometrial thickness less than 4 mm is associated with a low risk of endometrial carcinoma40–40; however, any focal endometrial lesion requires a biopsy. In contrast to postmenopausal women, the utility of transvaginal ultrasound is not well established in premenopausal women.41,42 There is no standard threshold for endometrial thickness in premenopausal women. Transvaginal ultrasound also does not appear to be an effective screening tool for women on hormone replacement therapy.43,44 Endometrial sampling is the gold standard for premenopausal women and women taking hormone replacement therapy. Endometrioid adenocarcinoma arises from the endometrium and is the most common pathological subtype (95% of cases) (Table 88-5). Uterine serous and clear cell carcinomas are less common adenocarcinomas but more aggressive and associated with a worse prognosis. Uterine sarcomas comprise 2% to 5% of uterine malignancies and arise from the myometrium or other mesenchymal elements (Table 88-6).45 Current classification guidelines for sarcomas include leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated endometrial sarcoma. Table 88-5 Classifications of Endometrial Carcinomas Table 88-6 Gynecologic Oncology Group Classifications of Uterine Sarcomas Endometrial adenocarcinomas appear to have two distinct mechanisms of pathogenesis.46 The most common adenocarcinoma, type I, arises in normal-functioning endometrium. Type I tumors tend to be associated with hyperplasia, be well differentiated, and express steroid hormone receptors. Type II adenocarcinomas arise in atrophic endometrium, are associated with endometrial intraepithelial carcinomas, and have a worse prognosis than type I tumors. Simple hyperplasia is the most common type and is a benign, diffuse thickening of the endometrium. Histologically, simple hyperplasia is characterized by dilated and increased numbers of endometrial glands, but minimal crowding or glandular complexity. Malignant transformation from simple hyperplasia is rare (Table 88-7). Complex hyperplasia is characterized by increased endometrial thickness because of increased numbers and crowding of the endometrial glands. The glands have irregular contours and markedly diminished stromal spaces. Complex hyperplasia without atypia is associated with a 3% rate of malignant transformation.47 Both simple and complex hyperplasia without atypia tend to regress with treatment. Table 88-7 Progression of Endometrial Hyperplasia From Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56(2):403–12. Atypical hyperplasia is characterized by an increased number of cellular atypia. Glandular morphology appears simple or complex and includes nuclear enlargement, increased nucleus-to-cytoplasmic ratio, nuclear hyperchromatism, prominent nucleoli, and loss of normal orientation of the epithelial cells. If atypia is present, the reported risk of finding an adenocarcinoma after hysterectomy ranges from 14% to 57%.48 Among such cancers, myometrial invasion is common, and up to 20% of cases will be grade II or III. Adenocarcinomas arising from hypertrophic endometrium or associated with atypical hyperplasia are typically endometrioid type. For this histologic subtype, the median age of diagnosis is 65 years. Seventy-five percent of uterine cancers are endometrioid adenocarcinoma in histology. Variations include villoglandular, secretory, ciliated cell, adenocarcinoma with squamous differentiation, and not otherwise specified (NOS). Adenocarcinomas are further categorized by depth of invasion and grade. International Federation of Gynecology and Obstetrics (FIGO) uses a combination of architectural patterns and nuclear features for histologic grading of tumors. Low-grade, or grade I, lesions are well-differentiated tumors composed of cells and glands that closely resemble those of the normal endometrium (Figure 88-1). Glandular growth patterns show 5% or less solid growth in grade I tumors. Grade II lesions contain 6% to 50% solid growth pattern, and grade III tumors are composed of more than 50% nonsquamous solid growth pattern.49 Although this system relies predominantly on the glandular architecture, FIGO recommends the overall grade of the tumor should be raised by one grade in cases with severe nuclear atypia. Higher-grade tumors are associated with increased incidence of lymph node metastasis, myometrial invasion, and a poorer overall prognosis.50 Depth of invasion is determined by measuring the percentage of cancer extension into the uterine wall, or myometrium. For staging purposes, FIGO classifies depth of invasion as less than 50% of the myometrium or greater than 50% of the myometrium. Uterine serous carcinomas represent 5% to 10% of endometrial malignancies. The median age at diagnosis is 63 years. Serous uterine carcinomas are not hormonally mediated and arise from endometrial intraepithelial carcinoma, a recognized precursor lesion (Figure 88-2). These cancers are considered high-grade with marked nuclear pleomorphism, multinucleated cells, psammoma bodies, hobnail cells, uneven gland borders, and papillary or solid growth patterns with slitlike spaces (Figure 88-3).51 Lymphovascular invasion is common and lymph node metastases are present in 36% of women with no myometrial invasion, 50% with less than one-half invasion, and 40% with greater than one-half invasion.52 Serous uterine cancers have a high rate of metastasis to the omentum and peritoneal surfaces.53 Survival rates are only 30% to 50% even when disease is confined to the uterus, and median survival is significantly shorter than that for endometrioid adenocarcinoma. In utero exposure to the synthetic estrogen diethylstilbestrol (DES) is associated with clear cell carcinomas of the uterus, vagina, ovary, and cervix. DES was prescribed to pregnant women between 1940 and 1971 for various indications, including prevention of miscarriage. The risk of developing clear cell carcinoma of the genital tract is 0.1% in a woman exposed to DES in utero.54 The median age of diagnosis is 66 years and clear cell carcinomas comprise 5% of adenocarcinomas.55 Cells form cystic solid or tubular cellular patterns whose cytoplasm appears clear or light pink on hematoxylin-eosin staining. Papillary formations, when present, often have a characteristic hyalinized core, and hobnail cells are frequently seen (Figure 88-4). Nuclear size varies, but high mitotic count is often present and lesions are considered high grade. Although pelvic failures occur in about one-third of patients postoperatively without adjuvant radiation, unlike serous carcinoma, this subtype has only a 15% rate of intraabdominal failure. Relapse in the lung, liver, and bone occurs in 25% of patients.56 The 5-year disease-free survival rate is approximately 40%. Uterine carcinosarcomas are highly aggressive tumors with a poor prognosis. These tumors were previously classified as uterine sarcomas and termed malignant mixed müllerian tumors; however, they are now classified as carcinomas arising from a monoclonal cancer cell that exhibits sarcomatous metaplasia. Uterine carcinosarcoma is rare, with an incidence of 1 to 4 per 100,000 in the United States in women older than age 35 years.57 African Americans have a twofold increase in incidence compared with non-Hispanic whites.58,59 A history of pelvic radiation exposure is associated with an increased risk of developing uterine carcinosarcoma.27,60 Uterine carcinosarcomas contain both sarcomatous and carcinomatous elements (Figure 88-5). The most common combination is a mixed homologous tumor consisting of an endometrial stromal sarcoma and a high-grade serous carcinoma. The carcinomatous or epithelial elements predominate metastatic sites and determine the overall course in advanced cases.61,62 Leiomyosarcoma makes up about 1% of uterine malignancies. Median age of diagnosis is 52 years. Leiomyosarcoma are thought to arise independently from leiomyomas (uterine fibroids). These tumors are characterized by abundant mitoses (greater than 10 per 10 high-power fields), prominent cellular atypia, and necrosis (Figure 88-6). The presence of two of the three features indicates a risk of metastasis greater than 10%.63 There are two variations of leiomyosarcoma: epithelioid leiomyosarcoma and myxoid leiomyosarcoma. Epithelioid leiomyosarcoma tumors are characterized by abundant eosinophilic cytoplasm, atypia, and >5 mitoses per 10 high-power fields. Benign pathology is not confirmed by the absence of coagulative necrosis. Myxoid leiomyosarcomas are highly malignant tumors characterized by a dense myxoid appearance.64 Stromal sarcomas have been divided into two categories: benign stromal nodule if the margins are smooth, and endometrial stromal sarcoma (ESS) if the margins are infiltrating or lymphovascular invasion is present (Figure 88-7). Previous classifications included low-grade sarcomas with fewer than 10 mitoses per 10 high-power fields, and high grade sarcomas with 10 or more mitoses per 10 high-power field.65 ESSs are malignant tumors composed of cells similar to those of the endometrial stroma. Histologically, the cells have scant cytoplasm and serpentine processes that infiltrate between muscle fibers and into lymphatic spaces. Mild nuclear atypia is present and mitoses are usually less than 10 per 10 high-power fields. Estrogen and progesterone receptors are generally expressed and are responsive to progesterone therapy.66 The tumors are characterized by long disease-free intervals; however, up to 36% of patients with stage I disease will recur. Adenosarcoma is a rare mixed tumor with a benign epithelial component and a malignant stromal element.68 These tumors generally have a low malignant potential and good prognosis. The majority of women present with stage I disease.69 Adenosarcoma with sarcomatous overgrowth is a variation of adenosarcoma and tends to have a worse prognosis.70 Type 1 and type 2 endometrial cancers demonstrate unique molecular pathways. Type 1 lesions are commonly associated with unopposed estrogen, endometrial hyperplasia and younger age. Type 1 tumors are thought to be a result of genetic alteration and hormones. Gene expression within the endometrium is closely regulated by systemic hormonal changes. As such, genes expressed in type 1 cancers resemble those of proliferative endometrium consistent with unopposed estrogen and endometrial proliferation. Loss of pentaerythritol tetranitrate (PTEN) function is thought to occur early during carcinogenesis. Up to 83% of endometrioid carcinomas demonstrate loss of PTEN.71 Mutations in microsatellite instability, K-ras, and β-catenin are also associated with type 1 endometrial cancers.72–76 Approximately 20% of sporadic endometrioid-type endometrial cancers demonstrate microsatellite instability. Nonendometrioid endometrial cancers are rarely associated with microsatellite instability. Both PTEN mutations and microsatellite instability have been demonstrated in precursor lesions such as complex atypical hyperplasia.71,77 In contrast, type 2 endometrial cancers are most commonly associated with mutation in p53, HER-2/neu amplification, and p16 inactivation. Up to 90% of serous carcinomas are associated with mutations in the tumor suppressor gene p53.74 Identification of genetic mutations has led to the development and study of targeted therapy for endometrial cancer. Prognosis has been correlated with several biological markers. Overexpression of p53, abnormal DNA ploidy, increased DNA methylation and expression of p21 has been associated with a worse prognosis; however, preserved expression of progesterone and estrogen receptors may indicate a more favorable prognosis. Endometrial adenocarcinomas express estrogen receptors (ERs) and progesterone receptors (PRs) in approximately 57% of cases, whereas 24% are negative for either receptor.78 Expression correlates with stage and tumor differentiation. Eighty percent of grade I tumors and 60% to 70% of grade II tumors express both ER and PR compared with 46% ER and 59% PR expression in grade III tumors.79 Higher-grade histologies, such as uterine serous carcinoma, also express lower levels of receptors compared with endometrioid carcinomas. Survival is better in ER-positive/PR-positive and ER-negative/PR-positive tumors compared with ER-negative/PR-negative or ER-positive/PR-negative tumors.80 ER status has been shown to be prognostic in some studies. Loss of PR expression is more common in metastatic tumors than loss of ER expression.81 Approximately 80% to 90% of women with endometrial cancer present with postmenopausal vaginal bleeding. In premenopausal women, endometrial cancer may present with abnormal bleeding patterns or menorrhagia. In either case, other malignancies such as cervical or vaginal cancer must be excluded. Bladder and gastrointestinal cancers can also present as irregular vaginal bleeding and should be evaluated accordingly.82 Less commonly, patients present with metastatic disease in the absence of vaginal bleeding. The severity of vaginal bleeding does not correlate with the risk of malignancy. Increasing age and vaginal bleeding is strongly associated with risk of malignancy. Postmenopausal bleeding, including spotting, is attributed to endometrial carcinoma in up to 20% of cases.35,36 Premenopausal women are less likely to be diagnosed with endometrial malignancy, but menorrhagia and menometrorrhagia should be evaluated as recommended by the American College of Obstetricians and Gynecologists.83 Endometrial cancer may present as abnormal cytology during routine pelvic examination with a pap smear. Atypical glandular or endometrial cells diagnosed on cervical cytology must be evaluated keeping in mind that malignant cells may arise from either the cervix or uterus. Advanced disease may present as pelvic pain, hematuria, hematochezia, renal obstruction, abdominal distention, or pulmonary symptoms. Endometrial carcinoma is surgically staged according to FIGO guidelines.84,85 In 1988, FIGO instituted surgical staging for endometrial cancer. Prior to this system, physical examination was used to clinically stage patients. In addition to hysterectomy, FIGO guidelines recommend the assessment of adnexa, pelvic, and paraaortic lymph nodes. In addition to revised endometrial carcinoma staging in 2009, FIGO implemented separate staging systems for uterine sarcomas (Tables 88-8 through 88-10). Table 88-8 FIGO 2009 Staging for Endometrial Carcinoma (Including Carcinosarcoma) Table 88-9 FIGO 2009 Staging for Leiomyosarcoma and Endometrial Stromal Sarcomas Table 88-10 FIGO 2009 Staging for Uterine Adenosarcoma The most recent classification system for endometrial carcinoma was changed to include only two substages of stage I. Stage IA tumors are limited to the inner one-half of the myometrium. Stage IB tumors invade one-half or more of the myometrium. Total hysterectomy with bilateral salpingo-oophorectomy with pelvic and paraaortic lymphadenectomy is the standard procedure for endometrial carcinoma.86 In contrast to prior endometrial carcinoma staging guidelines, intraperitoneal cytology is not part of FIGO 2009 staging. More than 50% of patients with positive peritoneal washings will have extrauterine disease involving the pelvis or abdomen; however, approximately 10% of FIGO stage I tumors will have positive peritoneal washings. One of the most important prognostic factors for uterine cancer is the presence of extrauterine disease. The approach to lymph node assessment in patients with early-stage disease is controversial. The rate of nodal involvement varies with stage and histology (Tables 88-11 and 88-12). Well-differentiated tumors with superficial invasion have a 3% to 5% risk of nodal metastasis, whereas deeply invasive poorly differentiated tumors have up to a 20% risk of nodal involvement.50,87 High-grade histology, such as serous or clear cell tumors, is also associated with increased risk of nodal metastasis. Myometrial invasion greater than one-half and tumors larger than 2 cm are also associated with an increased risk of nodal disease. There is no evidence that lymphadenectomy is beneficial in cases of uterine sarcoma. Table 88-11 Frequency of Positive Pelvic Lymph Nodes From Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105(2):109. Table 88-12 Frequency of Positive Paraaortic Lymph Nodes From Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105(2):109. Patients with disease confined to the uterus have an excellent prognosis compared with patients with extrauterine or metastatic disease. Current FIGO surgical staging guidelines more accurately predict survival than clinical staging.88 Stage I and II survival rates are approximately 80% to 90%, compared to 50% to 60% and 20% for stage III and IV disease, respectively. Myometrial invasion is the most important pathological feature influencing risk of lymph node metastasis. Twenty-five percent of deeply invasive (outer third of myometrium) tumors have pelvic lymph node involvement, compared with 1% of superficially invasive tumors, 5% of tumors involving the inner third, and 6% of tumors involving the middle third of the myometrium.50 Paraaortic lymph node metastasis are seen less often with 1% of superficial, 3% of inner third, 1% of middle third, and 17% of outer third tumors. Histologic grade correlates with depth of invasion, and more than half of grade 3 cancers are deeply invasive. Higher grade is also associated with lymph node metastasis. Grade 1 cancers carry a 3% risk of pelvic lymph node involvement, compared with 9% for grade 2 and 18% for grade 3 tumors.50 Endometrioid adenocarcinoma is the most common and most favorable histology. Uterine serous carcinoma and clear cell carcinoma are adenocarcinomas with a much higher propensity for nodal involvement and extrauterine spread, leading to a poorer overall prognosis. Uterine carcinosarcoma (previously called malignant mixed müllerian tumors) is also considered a high-risk variant of adenocarcinoma. Overall survival for endometrioid adenocarcinoma approaches 90%, compared with only 33% for other combined subtypes of adenocarcinoma.89 Traditionally, surgical staging was performed via laparotomy; however, minimally invasive surgery has gained wide acceptance as an alternative to laparotomy in the evaluation of gynecologic malignancies, in particular endometrial cancer. Minimally invasive surgery is associated with less postoperative pain, quicker postoperative recovery, and lower blood loss compared with traditional laparotomy.92–92 In addition, the use of laparoscopy for staging and treatment of endometrial carcinoma does not adversely affect recurrence or survival outcomes.93,94 A Gynecologic Oncology Group study randomly assigned more than 2600 patients with endometrial cancer to surgical staging via laparotomy or laparoscopy.93,94 The study concluded that laparoscopic surgical staging is a reasonable option with no differences in recurrence or survival. Patients who underwent laparoscopic surgical staging had fewer moderate to severe postoperative complications, equivalent detection rates of advanced-stage disease, and an improved quality of life through 6 weeks after surgery. Advances in surgical technology have introduced robotic-assisted surgical staging for endometrial cancer. The da Vinci surgical system was approved by the FDA for gynecologic use in 2005. The advantages of robotic-assisted surgery include 3D operative visualization, improved ergonomics during surgery, and seven degrees of freedom of articulation offering improved dexterity compared with laparoscopy. As in traditional laparoscopy, robotic-assisted surgical procedures are associated with lower blood loss, shorter hospital stays, and fewer perioperative complications compared to laparotomy.90,91 Long-term oncologic outcomes appear to be equivalent to traditional laparoscopy, but further follow-up is necessary.
Uterine Cancer
Introduction
Epidemiology
Stage
5-year Survival (%)
IA
90
IB
78
II
74
IIIA
56
IIIB
36
IIIC1
57
IIIC2
49
IVA
22
IVB
21
Etiology and Biological Characteristics
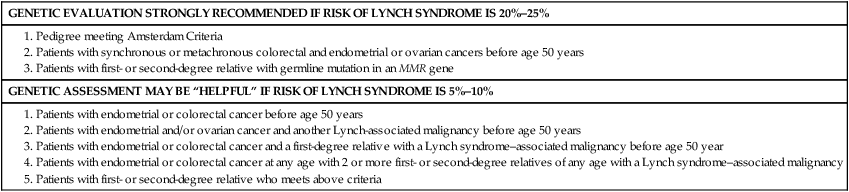
Genetics

GENETIC EVALUATION STRONGLY RECOMMENDED IF RISK OF LYNCH SYNDROME IS 20%–25%
GENETIC ASSESSMENT MAY BE “HELPFUL” IF RISK OF LYNCH SYNDROME IS 5%–10%
Risk Factors
Risk Factor
Relative Risk
Unopposed estrogen therapy
10-20
Tamoxifen
2.5
Polycystic ovarian syndrome
3-5
Obesity
2-5
Diabetes
2-3
Nulliparity
2-3
Estrogen-producing ovarian tumors
5
Previous Irradiation
Other Comorbidities
Protective Factors
Prevention and Early Detection
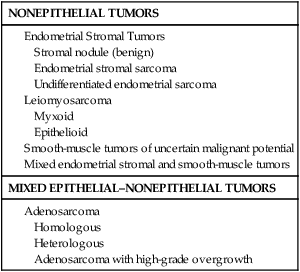
Pathology and Pathways of Spread
Pathogenesis Overview
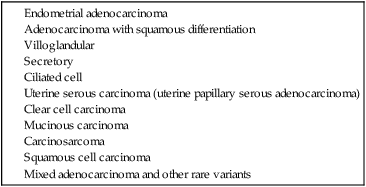
NONEPITHELIAL TUMORS
MIXED EPITHELIAL–NONEPITHELIAL TUMORS
Endometrial Hyperplasia
Histology
Progressed to Carcinoma (%)
Simple hyperplasia
1
Complex hyperplasia
3
Simple hyperplasia with atypia
8
Complex hyperplasia with atypia
29
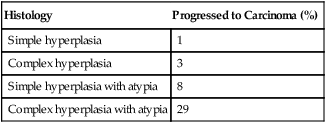
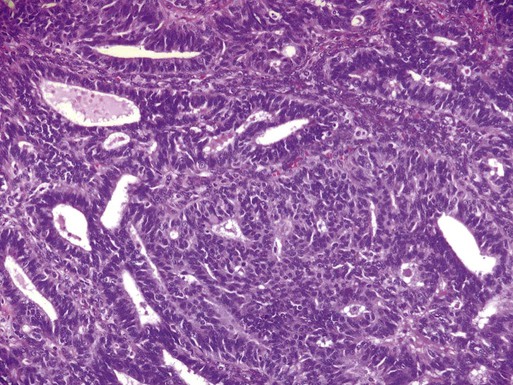
Endometrioid Adenocarcinoma
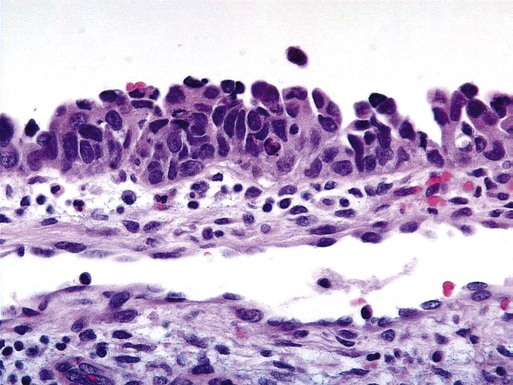
Uterine Serous Carcinoma
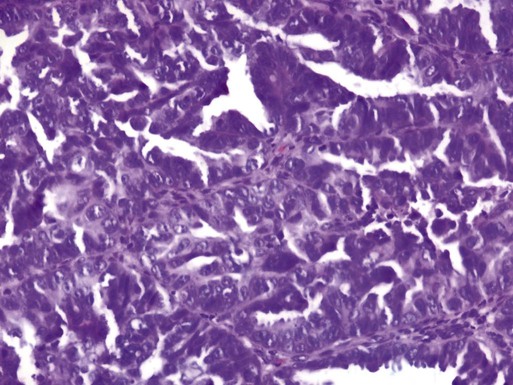
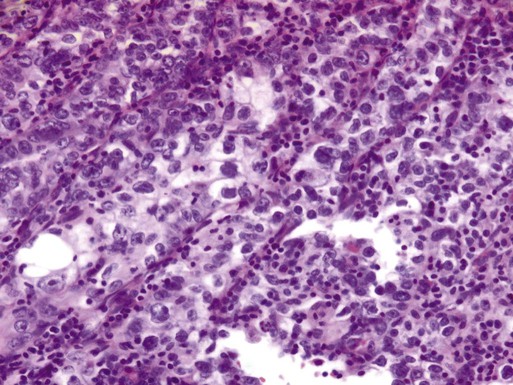
Clear Cell Carcinoma
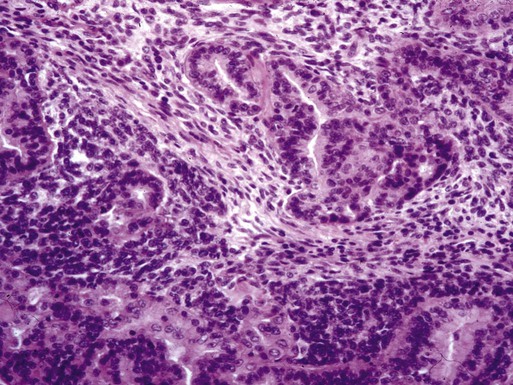
Carcinosarcoma
Leiomyosarcoma
Endometrial Stromal Sarcoma
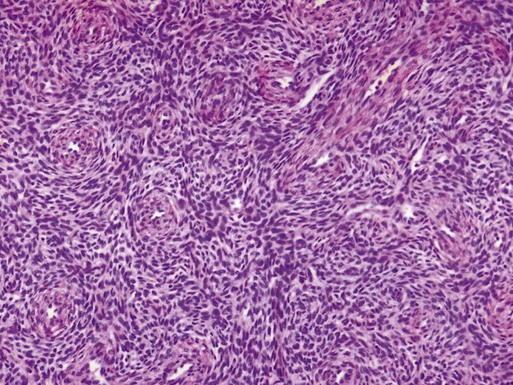
Mixed Epithelial–Nonepithelial Tumors
Molecular Pathways
Clinical Features
Staging
Stage
IA
Tumor confined to uterine corpus, <50% myometrial invasion
IB
Tumor confined to uterine corpus, ≥50% myometrial invasion
II
Tumor invades cervical stroma but confined to uterus
IIIA
Tumor invades uterine serosa or adnexa
IIIB
Involvement of vagina or parametrium
IIIC1
Metastasis to pelvic lymph nodes
IIIC2
Metastasis to paraaortic lymph nodes
IVA
Invasion of bladder or bowel mucosa
IVB
Distant metastases including intraabdominal metastasis and/or inguinal lymph nodes
Stage
IA
Tumor limited to uterus, size <5 cm
IB
Tumor limited to uterus, size >5 cm
IIA
Adnexal involvement
IIB
Extrauterine pelvic tissue involvement
III
Involvement of abdominal tissues
IIIA
1 site
IIIB
>1 site
IIIC
Metastases to pelvic and/or paraaortic lymph nodes
IVA
Tumor invades bladder or rectum
IVB
Distant metastases
Stage
IA
Tumor limited to endometrium
IB
Tumor limited to uterus, <50% myometrial invasion
IC
Tumor limited to uterus, ≥50% myometrial invasion
IIA
Adnexal involvement
IIB
Extrauterine pelvic tissue involvement
III
Involvement of abdominal tissues
IIIA
1 site
IIIB
>1 site
IIIC
Metastases to pelvic and/or paraaortic lymph nodes
IVA
Tumor invades bladder or rectum
IVB
Distant metastases
Depth of Myometrial Invasion
Grade 1 (%)
Grade 2 (%)
Grade 3 (%)
Endometrium only
0
3
0
Inner one-third
3
5
9
Middle one-third
0
8
4
Outer one-third
11
19
34
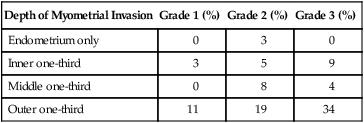
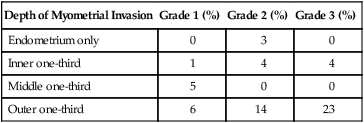
Depth of Myometrial Invasion
Grade 1 (%)
Grade 2 (%)
Grade 3 (%)
Endometrium only
0
3
0
Inner one-third
1
4
4
Middle one-third
5
0
0
Outer one-third
6
14
23
Stage
Depth of Invasion
Grade
Histologic Subtype
Therapy
Surgery as a Single Modality
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine