Sarcoma PDOX
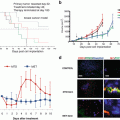
A patient high-grade undifferentiated pleomorphic soft-tissue sarcoma (UPS) from a striated muscle was grown orthotopically in the right biceps femoris muscle of nude mice to establish a PDOX model. Histological examination demonstrated eradication of the PDOX tumor with S. typhimurium A1-R followed by doxorubicin (DOX) [26] (Fig. 13.1a).
Follicular dendritic cell sarcoma (FDCS) is a rare and recalcitrant disease. A PDOX mouse model of FDCS was established in the biceps muscle of nude mice. The FDCS PDOX was resistant to both DOX and NVP-BEZ235, dactolisib (BEZ), a dual pan-phosphoinositide 3-kinase-mammalian target of rapamycin inhibitor. In contrast to DOX and BEZ, the FDCS PDOX was sensitive to S. typhimurium A1-R. The combination of S. typhimurium A1-R and either DOX or BEZ did not increase the antitumor efficacy of S. typhimurium A1-R, indicating that DOX and BEZ were not active in this PDOX model. S. typhimurium A1-R was effective in a PDOX model of FDCS. The patient failed first-line DOX therapy therapy, as did the PDOX model, which was sensitive to S. typhimurium A1-R [27] (Fig. 13.1b).
A PDOX model of osteosarcoma, established in the nude mouse distal femur with a lung metastasis, was resistant to CDDP as was the patient donor. The osteosarcoma PDOX model was sensitive to S. typhimurium A1-R administered intravenously (i.v.), but even more sensitive to S. typhimurium A1-R when administered intra-arterially, which caused regression of the tumor. Intra-arterial S. typhimurium A1-R was significantly more efficacious than when administered i.v. [57] (Fig. 13.1d).
A tumor from a patient with soft-tissue Ewing’s sarcoma, who failed DOX therapy, was implanted in nude mice to establish a PDOX model. The PDOX model faithfully replicated the DOX resistance the Ewing’s sarcoma had in the patient. S. typhimurium A1-R converted the Ewing’s sarcoma from DOX resistant to sensitive. S. typhimurium A1-R administered intra-tumorally (i.t.) arrested the growth of the Ewing’s sarcoma PDOX and S. typhimurium A1-R administered i.v. regressed the tumor [31] (Fig. 13.1e).
Melanoma PDOX
S. typhimurium A1-R highly and selectively colonized a PDOX melanoma and significantly suppressed tumor growth. The combination of Salmonella A1-R and cisplatinum (CDDP), both at low-dose, also significantly suppressed the growth of the melanoma PDOX [32], suggesting a role for S. typhimurium A1-R in combination therapy at low doses (Fig. 13.1f).
A melanoma, expressing the BRAF-V600E mutation, obtained from the right chest wall of a patient was grown orthotopically in the right chest wall of nude mice to establish a PDOX model. Temozolomide (TEM) combined with S. typhimurium was significantly more effective than either S. typhimurium A1-R alone or TEM alone. TEM combined with S. typhimurium A1-R could regress the melanoma in the PDOX model, again suggesting a role of S. typhimurium A1-R in combination chemotherapy [34] (Fig. 13.1c).
Pancreatic Cancer PDOX
A pancreatic cancer PDOX was transplanted by SOI in transgenic nude RFP mice so that the PDOX stably acquired RFP-expressing stroma for the purpose of imaging the tumor after passage to non-transgenic nude mice in order to visualize the tumor growth and drug efficacy [7, 29] (See Chap. 7 and 15). The nude mice with human pancreatic PDOX were treated with S. typhimurium A1-R or standard chemotherapy, including gemcitabine (GEM), which is a first-line therapy for pancreatic cancer, for comparison of efficacy. S. typhimurium A1-R treatment significantly reduced tumor weight, as well as tumor fluorescence area, compared to untreated control, with comparable efficacy of GEM, CDDP, and 5-fluorouracil (5-FU). Histopathological response to treatment was defined according to Evans’s criteria, which showed S. typhimurium A1-R had increased efficacy compared to standard chemotherapy [9].
A pancreatic cancer PDOX that was VEGF-positive and an orthotopic VEGF-positive human pancreatic cancer cell line (MiaPaCa-2-GFP) as well as a VEGF-negative cell line (Panc-1) were evaluated for sensitivity to S. typhimurium A1-R in combination with anti-angiogenic agents. Nude mice with these tumors were treated with GEM, bevacizumab (BEV), and S. typhimurium A1-R. BEV/GEM followed by S. typhimurium A1-R significantly reduced tumor weight compared to BEV/GEM treatment alone in the PDOX and MiaPaCa-2 models. Neither treatment was as effective in the VEGF-negative model as in the VEGF-positive models. These results demonstrate that S. typhimurium A1-R following antiangiogenic therapy is effective on pancreatic cancer including the PDOX model [11].
PDOX Models to Determine Efficacy and Distinguish Similar Molecular-Targeting Drugs
Ewing’s sarcoma is a rare and aggressive malignancy. A chest-wall metastasis from a patient with Ewing’s sarcoma with cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) loss and FUS-ERG fusion was implanted in the right chest wall of nude mice to establish a PDOX model. Tumor growth was significantly suppressed by CDK 4/6 inhibitor (palbociclib) and an IGF-1R inhibitor (linsitinib). In contrast, first-line therapy DOX did not inhibit tumor growth at any time point, which is consistent with the failure of DOX in the patient. The PDOX model identifies effective targeted molecular therapy of a recalcitrant DOX-resistant Ewing’s sarcoma with specific genetic alterations [28].
The BRAF-V600E-mutant melanoma PDOX grown orthotopically in the right chest wall of nude mice described above was also tested with the molecularly targeting drug. Trametinib (TRA), an MEK inhibitor, was the only agent of the four tested that caused tumor regression. In contrast, another MEK inhibitor, cobimetinib (COB), could slow but not arrest growth or cause regression of the melanoma. First-line therapy temozolomide (TEM) could slow but not arrest tumor growth or cause regression. The BRAF-V600E-mutant melanoma was a candidate for vemurafenib (VEM) since VEM targets this mutation. However, VEM was not effective. The PDOX model thus helped identify the very high efficacy of TRA against the melanoma PDOX which was superior to COB even though they both target MEK. Genomic analysis alone was not sufficient for determining drug efficacy as the PDOX failed VEM and responded well to one MEK inhibitor and not another [33].
Use of PDOX Models to Evaluate Anti-metastatic Agents
We evaluated the efficacy of zoledronic acid (ZA) on primary tumor growth and metastasis in a PDOX nude mouse model of pancreatic cancer. ZA alone did not significantly suppress tumor growth. The primary tumor weight of GEM + ZA-treated mice was significantly decreased compared to GEM alone-treated mice. No metastasis was detected in combination GEM + ZA-treated mice compared to the control group. In contrast, GEM alone could not suppress metastasis. The results indicate that ZA can selectively target metastasis in a pancreatic cancer PDOX model [12].
A subcutaneous nude mouse model of HER-2-expressing cervical carcinoma was not sensitive to entinostat (a benzamide histone deacetylase inhibitor). Entinostat also did not inhibit primary tumor growth in a PDOX model of this tumor. However, in the PDOX model, entinostat alone significantly reduced the metastatic tumor burden, compared to the control. Thus, only the PDOX model could be used to discover the antimetastatic activity of entinostat for this tumor. The results indicate the importance of using mouse models that can recapitulate metastatic cancer for precisely individualizing cancer therapy [19].
Materials and Methods
Mice
Use athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6-weeks-old. Conduct all animal studies with an AntiCancer Institutional Animal Care and Use Committee (IACUC) protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1.
Preparation and Administration of S. typhimurium A1-R
GFP-expressing S. typhimurium A1-R bacteria (AntiCancer, Inc., San Diego, CA, USA) were grown overnight on LB medium, and then diluted 1:10 in LB medium. The bacteria were harvested at late-log phase, wash with PBS, and then diluted in PBS. S. typhimurium A1-R was administered intratumorally or intravenously in PBS to each tumor [9, 11, 26, 27, 32, 34].
Culture of GFP-Labeled S. typhimurium A1-R Bacteria from Tumor and Normal Organs
PDOX tumor tissues as well as normal organs (liver, spleen, and blood) were minced and diluted in 1:1, 1:10 and 1:100 with 100 μL PBS, respectively. Each dilution (10 μl) were spotted on an LB agar plate containing 50 μg/mL ampicillin, and incubated at 37 °C for 24 h. Colonies are visualized by GFP expression and quantitated per mg of tissue [32].
Imaging of S. typhimurium A1-R in Tumors
Histological Analysis
Tumor samples in 10% formalin were embedded in paraffin before sectioning and staining. Tissue sections (5 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H & E) staining was performed according to standard protocol [26].
References
1.
2.
3.
Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically from histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–9.CrossrefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree