INTRODUCTION
Urologic malignancies account for more than 40% of all new cancer diagnoses and 15% of all cancer deaths in the United States (
1). In addition to these primary sites of disease, metastases from other malignancies and the side effects of the surgery, radiation, and chemotherapy used to treat them can all deleteriously impact the genitourinary system. This chapter will review many of the most common urologic issues cancer patients experience and provide a schema for their evaluation and management.
It is our belief that palliative care and supportive oncology should be incorporated into the treatment plan early, if not from the time of diagnosis, for many patients. The work of the American Urological Association’s web-based ethics curriculum, American College of Surgeons’ ethics curriculum and guide to surgical palliative care, and Northwestern University’s Education in Palliative and End-of-Life Care program has increased surgeon awareness regarding the importance of interventions that are designed to improve patients’ quality of life (QOL) even though they may not prolong survival (
2,
3,
4). However, two recent publications have shown that early supportive oncology involvement improves QOL, which is not surprising, but that structured palliative care in addition to routine cancer treatment can actually improve survival when compared with standard oncologic therapy alone, which will certainly stimulate further research on the possible synergy between curative and supportive care (
5,
6).
SURGICAL PALLIATIVE CARE
Just as palliative care has evolved in scope and practice within the field of internal medicine, the concept of surgical palliative care has matured along with it (
7). The definition of surgical palliative care can now be understood as any procedure whose primary intent is to improve QOL or mitigate symptoms caused by advanced disease (
2). The efficacy of surgical palliation should be evaluated by the magnitude and duration of improvement in patient-reported symptoms (
2). The American College of Surgeons has outlined three key components that must be addressed prior to undertaking a palliative intervention. These include (i) understanding the patient’s symptoms and goals of care, (ii) estimating the likely impact the proposed intervention will have on the patient’s symptoms, and (iii) the patient’s prognosis and trajectory of disease (
2). While not an exhaustive list, using these points to frame discussions with patients and their families about potential procedures/surgery can help develop realistic expectations, respect patient autonomy, and avoid the harms of unnecessary surgery (
7,
8). Another specific issue that should be discussed prior to palliative surgery is whether an advance directive such as a do-not-resuscitate (DNR) order has been completed, and how it should be handled during the perioperative period (
9).
The American Society of Anesthesiologists, American College of Surgeons, and the Association of Operating Room Nurses have stated that it is inappropriate to automatically discontinue a patient’s DNR order upon entry into the operating room (
9). Instead, they advocate for “required reconsideration” when a patient with a DNR needs a surgical intervention. This allows the patient, surgeon, and anesthesiologist to review the goals of care and proposed treatment plan in order to determine the best course of action. The DNR order may then be maintained, suspended, or revised during the perioperative period. Advance directives are reviewed in detail in
Chapter 57 of this textbook.
URINARY OBSTRUCTION
Obstruction of the urinary tract can occur due to a wide range of pathophysiology. It can be chronic resulting in the slow deterioration of renal function, but it can also be acute resulting in significant discomfort and life-threatening illness. Common causes of upper urinary obstruction include kidney and ureteral stones, strictures of the ureter, and extrinsic compression of the ureter from abdominal or retroperitoneal masses. Lower urinary tract obstruction may be due to benign prostatic obstruction, bladder stones, urethral strictures, and extrinsic compression from pelvic masses. Naturally, upper urinary tract obstruction has a different evaluation and treatment algorithm compared with lower urinary tract obstruction.
The etiology of obstruction may be secondary to prior disease treatment, the consequence of progressive disease, or even age-related pathophysiology unrelated to the primary diagnosis. Keeping these factors in mind, treatment plans should be individualized for each patient after taking into consideration life expectancy, anesthesia risk, and social support
systems. As with any medical workup, the first step is still a detailed history and physical examination to assess the possible location(s) and acuity of the obstruction. The treatment of urinary tract obstruction is variable, and the treatment of choice may change as the patient’s goals of care evolve (
Table 33.1).
Upper Urinary Tract Obstruction
Acute obstruction of the upper urinary tract typically presents with classic symptoms such as flank pain, dysuria, and hematuria. These symptoms can be elicited by intrinsic and extrinsic obstructive processes. Intrinsic obstruction can be caused by urinary stones, blood clots, and strictures, whereas extrinsic obstruction can occur when any abdominal or pelvic structure compresses the ureter or renal pelvis. Common causes of extrinsic obstruction include tumors, fibrosis, and enlarged lymph nodes.
Imaging is a mainstay in the evaluation of upper tract obstruction. Dilatation of the renal pelvis/ureters and intrarenal stones can be visualized very well on ultrasound; however, identifying the cause of obstruction below the renal pelvis may be difficult because the entire length of the ureter may not be well visualized with ultrasound alone. The quality of the images is also dependent on patient’s body habitus as well as the skill of the technician. Abdominal x-ray without the use of intravenous contrast has virtually no value in evaluating upper urinary tract obstruction. An intravenous pyelogram (IVP), which consists of serial abdominal x-rays shot in a timed fashion after the injection of intravenous contrast, helps delineate the renal shadow and the drainage patterns of the ureters. The IVP was the standard radiologic evaluation for the upper urinary tract until recently but has been supplanted by computed tomography (CT) urogram or CT-IVP due to increased sensitivity as well as additional anatomic information provided by the CT images.
A CT urogram consisting of a non-contrast CT scan followed by a CT scan with intravenous contrast in three phases (arterial, venous, and delayed) is now considered the standard of care by most urologists. Urolithiasis and renal masses are readily identified and characterized, a basic assessment of renal function can be done, and filling defects within the renal pelvis and ureters can be seen. The primary limitation of CT urography in this patient population is the prevalence of renal insufficiency/failure, which often precludes the use of intravenous contrast.
Magnetic resonance imaging (MRI) delineates soft tissue better than CT but tends to be used more as a secondary test to clarify questions posed by the original scan (
10). MRI is the study of choice when evaluating for tumor involvement in the renal vein or vena cava (
11). Magnetic resonance urography (MRU) also allows for visualization of all anatomic components of the urinary tract using heavily T2-weighted sequences or gadolinium-enhanced T1 images. Since there is no ionizing radiation, it is especially useful for pediatric and/or pregnant patients. However, there are a few disadvantages to MRU that keep it from being a first-line test. MRU is still limited in its ability to visualize stones; compared with CT the availability of the test itself is limited; the time needed to perform an MRU is significantly longer than a CT urogram (30 to 60 minutes versus 10 to 15 minutes, respectively) (
10).
After obtaining a detailed history, general lab work (serum chemistries and urine analysis), and appropriate imaging, urologic consultation may be needed for a more in-depth evaluation and treatment plan. More invasive evaluation with cystoscopy/ureteroscopy may be warranted if a questionable filling defect were to be seen on delayed CT images for example.
Non-surgical Treatment
The treatment of extrinsic compression of the upper urinary tract will vary depending on the etiology, symptoms, and the effect on renal function. If caused by extrinsic compression from malignancy, the primary goal is to treat the underlying disease when possible. Obstruction due to a primary genitourinary malignancy may be treated with either local surgical treatment or systemic therapy, depending on the stage of disease. Obstruction caused by iatrogenic injury or treatment side effect can be temporized with a percutaneous nephrostomy tube or ureteral stent until the inciting factor resolves or definitive surgical treatment/repair is safe to attempt.
Surgical Treatment
Internal ureteral stenting has long been a mainstay for the treatment of upper tract obstruction. Urinary stents are not permanent; and, if left in too long, stents may become encrusted and eventually fail. Traditionally, urologists have recommended replacing stents every 3 to 6 months. It is preferred to place ureteral stents endoscopically in a retrograde fashion in the operating room under general anesthesia. Chung et al. described their 15-year experience with internal ureteral stents for management of extrinsic obstruction and showed a 40.6% failure rate within the first 11 months. Predictors of stent failure include a diagnosis of cancer (regardless of type), baseline creatinine more than 1.3 mg/dL, and post-stenting radiation or chemotherapy (
12).
The Resonance metallic ureteral stent (Cook Medical, Bloomington, IN, USA) is a stent designed for the longterm management of extrinsic ureteral obstruction and may remain in place up to 12 months. The metal stent has greater tensile strength than plastic stents and does not compress as readily. Liatsikos et al. performed a prospective study of 50 patients, consisting of both malignant extrinsic obstruction and intrinsic stricture disease, and patients with malignant extrinsic obstruction showed patency rates of 100% with a mean follow-up time of 8.5 months, while patients with intrinsic stricture disease had a patency rate of 44%. Failure was noted within the first 2 weeks in the latter group (
13).
Thermo-expandable stents such as the Memokath (PNN Medical, Denmark) have also been developed as an alternative solution for the long-term management of ureteric obstruction. Agrawal et al. performed a prospective study on 55 patients, a mix of malignant extrinsic compression and intrinsic stricture disease, who had a Memokath placed with a mean follow-up of 16 months (range 4 to 98). Fourteen patients required reinsertion over a mean of 7.1 months for migration, encrustation, stricture progression, or incorrect length. The remaining patients maintained their stents between 8 and 12 months and then they were routinely replaced (
14). Like the Resonance and Memokath stents, many new innovations in ureteral stents are in development, but the long-term efficacy of all stents is not well defined and more data are needed.
Percutaneous nephrostomy tubes drain urine directly from the kidney through the patient’s back and into an external collection device. These tubes are often placed under local anesthesia with sedation by an interventional radiologist under ultrasound guidance, with fluoroscopy, or CT guidance for the most difficult cases. However, patients will be required to be prone for the procedure which may be difficult in elderly and obese patients and those with respiratory compromise. Like ureteral stents, nephrostomy tubes should be exchanged every 3 to 6 months. The advent of external/internal drainage systems (nephroureteral stents) have allowed for temporary nephrostomy tube placement with the ability to later convert to anterogradely placed internal ureteral stents.
In acute obstruction with clinical signs of sepsis (e.g., fever, tachycardia, and hypotension), percutaneous nephrostomy tube placement is the recommended intervention. Retrograde instrumentation with cystoscopy and ureteral stent placement in the setting of an active infection puts patients at significant risk for urosepsis, which can be lifethreatening (
15). Other times, nephrostomy tubes are used as a last resort after failure of attempts to place a ureteral stent endoscopically. Ku et al. described a recent retrospective analysis of complications in 148 patients with malignant extrinsic ureteral obstruction who underwent either nephrostomy tube or ureteral stent placement. The study noted no significant differences in fevers, acute pyelonephritis, or catheter-related complications between the two groups (
15). When considering primary palliative treatment for obstruction, the risks of anesthesia and the procedure itself as well as the impact of the nephrostomy tube/stent on QOL must be discussed with the patient or his/her health care proxy.
Given the fact that nephrostomy tubes involve direct puncture of the kidney, there is a small risk of bleeding, which is not seen in ureteral stenting. A review of a singlecenter experience with 500 percutaneous nephrostomy tube placements revealed a major complication rate of 0.45% and a minor complication rate of 14.2% (
16). Major complication was defined as gross hematuria and hemodynamic instability requiring surgical intervention and/or blood transfusion. Minor complications were tube complications (e.g., dislodgement and kinking) or gross hematuria for greater than 48 hours without clinical symptoms. Given the risk of bleeding, an anti-coagulated or thrombocytopenic patient is rarely a candidate for immediate diversion with a percutaneous nephrostomy tube.
There are not many studies comparing QOL after nephrostomy tube placement and after ureteral stenting. The percentage of patients describing their stents as “terrible” or having a “serious impact on daily life” is as high as 66% in some QOL studies (
17). QOL studies using standardized methods for nephrostomy tubes are few. Of the studies that do exist, the most common complaints involve tube dislodgement, urine leakage around tube, and skin excoriation at the tube exit site (
17). Although hard to objectively measure, the concept of extra medical devices (e.g., nephrostomy with external drainage bag) is anecdotally always a concern to patients and their caregivers. Ultimately, the decision for ureteral stent versus percutaneous nephrostomy will be unique to each patient.
In general, surgical treatments for obstructive uropathy in a palliative care setting are temporizing measures and aggressive surgical approaches are generally not indicated. However, advanced prostate and/or bladder cancer can cause obstruction due to local tumor extension occluding the distal ureters. In cases where it is difficult to pass internal stents, in percutaneous nephrostomy tube failure, or in QOL choice by the patient, pelvic exenteration can be considered. Palliative exenteration is a poorly studied area, but several small series have reported 5-year life expectancy ranging from 25% to 40% in well-selected metastasis-free patients (
18). Select palliative exenteration patients show life expectancy ranging from 18 to 24 months with improved QOL (
18). Given
the morbidity of any exenterative surgery, it is important to ensure that the patient has a good estimated life expectancy. In addition, patients should be counseled extensively on the long recovery process, high likelihood of perioperative complications, and no guarantee of extending life or improving QOL.
Lower Urinary Tract Obstruction
Stones in the lower urinary tract are far more common in men due to prostatic outlet obstruction leading to elevated residual urine in the bladder, although women can also develop them. Advanced prostate cancer can cause bladder outlet obstruction as well, regardless of the actual overall size of the prostate. Transitional cell tumors of the bladder can obstruct outflow as well. Urethral stricture disease is more common in patients who have had long-term urinary catheters, recurrent cystoscopy, sexually transmitted infections, or pelvic trauma.
The male lower urinary tract is generally defined as the bladder, bladder neck, prostatic urethra, and the penile urethra. A detailed medical history, including past surgeries, plus a validated questionnaire (e.g., International Prostate System Score) will be the basis for evaluating obstruction of the lower urinary tract. While always recommended, a digital rectal examination (DRE) assessment of “prostate size” does not always correlate with symptoms of obstruction. “Small” prostates on DRE can still cause significant obstruction due to anatomic variations such as an enlarged prostatic median lobe, which can act as a ball valve causing outlet obstruction.
The female lower urinary tract has a much shorter urethra due to the lack of a prostate; consequently, lower urinary tract obstruction in females is far less common. Extrinsic compression of the bladder from pelvic malignancies and pelvic organ prolapse can cause dysfunctional voiding such as incomplete emptying, urinary frequency and urgency, and incontinence. Iatrogenic causes such as pelvic/vaginal/urologic surgery in the past can also cause lower urinary tract obstruction, which reinforces the need for a detailed history. Therefore, a pelvic speculum examination is a key component to the physical assessment. Abdominal ultrasound is a safe and effective way of evaluating lower urinary tract obstruction. Using ultrasonography, a post-void residual can be calculated and larger bladder stones can be imaged. Urinalysis can also help rule out the presence of infection, which in itself can exacerbate symptoms of lower urinary tract obstruction. A serum creatinine will also help assess overall renal function, which can dictate the type and timing of treatment. These basic evaluations should be completed prior to urologic consultation for urinary obstruction.
Non-surgical Treatment
Benign prostatic hyperplasia (BPH) will affect many men as they age. Maximal medical therapy, as defined in the Medical Therapy of Prostatic Symptoms trial, found significant improvement in American Urological Association symptom scores when patients were given a combination of α-blockade (doxazosin) and 5-α-reductase inhibitor (finasteride) together compared with either drug alone. The study was double blinded with a mean follow-up of 4.5 years and involved 3,047 patients (
19). If a patient is assessed by DRE or ultrasound and is found to have a prostate larger than 30 g, combination therapy with an α-blocker and 5-α-reductase inhibitor (e.g., finasteride and dutasteride) can be instituted (
20). It should be noted that the effects of α-blockers are often appreciated within several days, whereas 5-α-reductase inhibitors take months to improve urinary symptoms. Additionally, 5-α-reductase inhibitors decrease serum prostate-specific antigen (PSA) values, and the use of this medication must be taken into account for men undergoing prostate cancer screening, although screening may no longer be appropriate for many men in the palliative care/supportive oncology population (
21).
Although medical therapy significantly improves the voiding function of many patients, its effects are limited. Many patients with voiding difficulties are older and may have baseline mobility issues. This may be especially true of patients with advanced malignancy who have decreased performance status and spend considerable time in bed. Normal voiding function is difficult to achieve in a recumbent position.
When manual dexterity is preserved, clean intermittent catheterization (CIC) is a reasonable option. First described by Lapides et al. (
22) in 1971, CIC education and training allows patients to empty their bladder on a schedule. This is still the simplest form of intervention for outlet obstruction. The key caveat is that patients are required to have the mental and manual dexterity to be able to do this for themselves or they should have a reliable caregiver assume this responsibility.
When a patient is not a candidate for CIC, an indwelling urinary catheter is a very common intervention for lower urinary tract obstruction. A semi-permanent catheter creates a pathway for bacteria to enter the urinary tract; therefore, bacteriuria is a common problem in chronically catheterized patients. If asymptomatic, treatment for bacteriuria is usually discouraged to avoid the creation of drug-resistant organisms (
23). Indwelling catheters should routinely be changed every 10 to 12 weeks (
23). Oftentimes, the immobilized patient may benefit most from an endoscopically placed suprapubic catheter. By removing the catheter from the urethra, the risk of urethral erosion or stricture is decreased and general comfort is improved.
Surgical Treatment
Surgical approaches to bladder outlet obstruction have become varied over the years but the gold standard is still the traditional electrosurgical transurethral resection of the prostate (TURP). Although the approach is endoscopic, a TURP is not without morbidity. Irrigation solutions such as glycine or mannitol were used in the past putting patients at risk for dilutional hyponatremia (TUR-syndrome) if too much fluid was absorbed through open venous sinuses during resection. Today, most cases are done using a bipolar resectoscope and
normal saline, but a patient can still become fluid overloaded from the absorption of irrigant (
24). As with any surgical procedure, anesthesia risk and blood loss are always concerns as well. Caution still needs to be used when considering TURP for patients with significant cardiopulmonary history.
Specific to the palliative care setting, the concept of the “channel TURP” has become more popular. Rather than subjecting patients to complete resection, which increases the risk of morbidity, the goal of the “channel TURP” is to remove enough tissue to allow successful bladder emptying while minimizing the amount of time spent under anesthesia and the amount of fluid absorbed by the patient during the procedure. Patients with significant comorbidities or even with prostate cancer that may be invading the bladder can be considered candidates for a channel TURP. Marszalek et al. (
25) described a rate of 25% repeat TURP, 11% requiring permanent catheters, and 10% with some incontinence in a series of 89 prostate cancer patients who underwent a “channel TURP.”
Laser photovaporization of the prostate (PVP) is one of the newer tools at the disposal of urologists for managing benign prostatic hyperplasia (BPH). The “greenlight laser” (AMS Medical Systems) energy is selectively absorbed within tissue by hemoglobin. This selective absorption improves the hemostatic effect of the laser while vaporizing prostatic tissue. Multiple studies have shown that the PVP compares favorably with the TURP with significantly less blood loss and shorter catheterization time (
26).
The improvement in blood loss has led many urologists to expand the indications of PVP use. With many older patients on oral anticoagulant or antiplatelet therapy, PVP is being used to relieve bladder outlet obstruction in patients who are not candidates for TURP due to increased risk of bleeding. Woo and Hossack recently described their experience with PVP in 43 men who were on Coumadin during their procedure. No patient needed a blood transfusion and only six (14%) patients required a catheter for more than 24 hours (
27). In the palliative care and supportive oncology population, PVP is a reasonable option to consider in patients who may have more significant medical comorbidities or require long-term thromboprophylaxis due to blood clots associated with malignancy.
HEMATURIA
While there are many benign causes of hematuria, including glomerulonephritis, cystitis, renal trauma, BPH, kidney, ureteral and bladder stones, radiation cystitis, and bacterial and viral infections, hematuria, whether gross or microscopic, may also be an indicator of a malignant process within the urinary collecting system. Therefore, a thorough investigation of the entire upper and lower urinary tract should always be considered. The two most common tests used for the detection of blood in the urine are the urine dipstick and microscopy. Urine dipsticks can have false-positive reactions in the presence of hemoglobin or myoglobin; consequently, when a dipstick is positive, microscopy is usually necessary to verify the presence of red blood cells (RBCs) and assess RBC morphology.
Once hematuria is confirmed, then a urine culture should be done to eliminate infection as the etiology of bleeding. In the absence of infection or after resolution of infection, a second positive urinalysis/microscopy would indicate the need for a complete urinary tract evaluation (
28). If an MRI urogram or CT urogram is performed adequately, then the evaluation is complete after cystoscopy. However, if an ultrasound or crosssectional imaging with incomplete or absent delayed phase images of the upper urinary tract is used for evaluation, then cystoscopy with bilateral retrograde pyelograms is indicated.
Gross hematuria refers to the presence of blood in the urine that can be seen with the unaided eye. It is important to confirm the presence of RBCs once dark urine is seen. There are multiple other potential causes of discoloration within a urine specimen. Some of the more common causes of discolored urine include concentrated urine and systemic administration of flutamide, phenazopyridine, sulfasalazine, phenolphthalein (seen with the use of some over-the-counter laxatives), nitrofurantoin, metronidazole, methylene blue, bilirubinuria, and vitamin B complex. When a patient presents with a history of gross hematuria and no evidence of infection, the hematuria workup may be done without confirmatory testing of the urine. Anticoagulated patients with hematuria should not be excluded from the workup since the anticoagulation may unmask a previously unidentified lesion in as many as 25% of patients (
29).
Causes of asymptomatic microscopic hematuria (
Table 33.2) range from minor, clinically insignificant findings that require no intervention to lesions that could be life threatening. When presented with a patient with asymptomatic hematuria, it is important to distinguish whether the bleeding originates from the lower urinary tract (urethra/prostate/bladder) or the upper urinary tract (ureters/kidneys). Often the history and physical examination may identify the location of the hematuria.
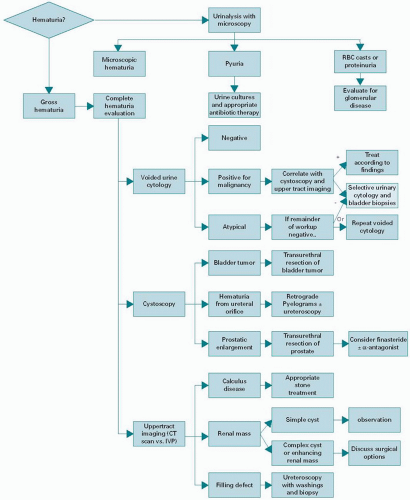
The appropriate evaluation (
Figure 33.1) includes a history and physical examination, laboratory analysis of the urine, upper tract evaluation of the ureters and kidney with a CT, lower tract evaluation by cystoscopy, and urine cytology. A flexible cystoscopy can be performed in the office by most urologists. With a thorough cystoscopy, the urethra, prostate, bladder, and ureteral orifices can be carefully examined and the source of bleeding often identified. Observation of the ureteral orifices may demonstrate bloody efflux from one side, further aiding in the identification of the laterality of an upper urinary tract source. In patients with microscopic hematuria, as many as 16% will have a malignancy identified through this evaluation and 3% of patients with a negative hematuria evaluation will have a malignancy found on a subsequent workup (
31). Lateralizing hematuria requires a retrograde pyelogram or delayed imaging with CT to properly visualize the ureters. The finding of a filling defect may necessitate ureteroscopy and fulguration, biopsy, or resection, as well as the treatment of non-malignant causes of upper tract hematuria.
When symptomatic, lower urinary tract bleeding may present with dysuria, frequency, urgency, lower abdominal pain, and urinary retention. As the symptoms can sometimes be severe, it is important to treat them while awaiting results from the urine tests. When lateralizing hematuria is found on cystoscopy, one should begin with a radiographic study. Retrograde pyelography or delayed imaging of an abdominal CT scan with oral and IV contrast may be sufficient to identify filling defects and masses in the ureters and kidneys.
Symptomatic upper tract hematuria usually presents with lateralizing flank or abdominal pain caused by obstruction, or if brisk bleeding from the upper tract is present, the patient may also have lower urinary tract symptoms and urinary retention secondary to clots.
Management of Lower Urinary Tract Hematuria
The initial management of all hematuria is dependent on the patient’s hemodynamic stability. When hemodynamically unstable, the patient should be aggressively hydrated, transfused, and coagulopathies reversed as needed. Once stabilized, the source of bleeding can be found and treated. If the patient is stable and bleeding is not profuse, the patient may be allowed to hydrate themselves to dilute the blood in their urine and should limit physical exertion as that may exacerbate bleeding. A careful medication review for non-steroidal anti-inflammatory drugs, aspirin, antiplatelet agents, lowmolecular weight heparins, warfarin, and other drugs that can potentiate bleeding is also critical. These should not be stopped until the reason for their use and the risk of withdrawing them have been carefully determined. A summary of management of gross hematuria is shown in
Table 33.3.
When lower urinary tract obstruction secondary to clots occurs, a large-bore three-way catheter should be placed and the bladder aggressively hand irrigated to remove all clot. Once the clots are removed, continuous bladder irrigation (CBI) may be initiated in order to prevent further clot formation. Alternatively, the patient may be observed without CBI if the resulting urine is clear, indicating that the bleeding was self-limited. Should persistent catheter obstruction occur due to recurrent or persistent clots, a Couvelaire catheter can be used as these catheters have a larger channel through which to hand irrigate out large volumes of clots and are therefore less likely to obstruct. If these measures fail, then the patient may be taken to the operating room for clot evacuation and resection or fulguration of the bleeding source.
Once the conservative treatment of hematuria fails, there are several adjunctive treatments that may be used for protracted bleeding in the bladder. If the bleeding is from the prostate, placing a Foley catheter with a 30 cc balloon on gentle traction may be sufficient to tamponade the bleeding. Alternatively, 5-α-reductase inhibitors have been used and have been shown to be effective in reducing bleeding (
32). For severe cases of prostatic bleeding, transurethral fulguration or resection of the prostate can be effective in halting the bleeding. ε-Aminocaproic acid, an inhibitor of fibrinolytic enzymes like plasmin, can be given intravenously at a loading dose of 5 g followed by hourly doses of 1.00 to 1.25 g. Once the patient responds, usually within 6 to 8 hours, the drug can be dosed and administered orally. Alternatively, ε-aminocaproic acid can be given intravesically as 200 mg/L of normal saline solution through CBI, which is continued for 24 hours after the bleeding has stopped. This approach has an observed resolution of hematuria in 91% of the cases (
33). It should be employed with great caution since an increase in thromboembolic events
has been observed and the formation of large intravesical clots is a risk when ε-aminocaproic acid is used (
34).
Other intravesical agents commonly used are alum, silver nitrate, and formalin. Alum precipitates proteins and may quickly stop bleeding by forming clots at the source of bleeding. It is important to minimize clot formation in the bladder when using alum as it also forms large amorphous clots that are difficult to remove even endoscopically (
35). Serum aluminum levels should be monitored in patients with renal insufficiency, as high levels may cause encephalopathy secondary to acute neurotoxicity. Treatment of acute systemic aluminum toxicity involves chelation with deferoxamine (
36). An intravesical solution of 0.5% to 1.0% silver nitrate causes chemical coagulation at the bleeding sites but may also cause renal failure in patients with reflux, necessitating a pre-treatment cystogram to assess for the presence of vesicoureteral reflux (
37). Formalin can also be used in cases of severe, intractable hematuria. It permanently precipitates the bladder proteins as well as proteins in the small vessels of the bladder. This may cause fibrosis, and in some cases a small-capacity bladder. It may cause severe renal injury in patients with vesicoureteral reflux, so pre-treatment cystogram is again mandatory (
38). Intravesical formalin is painful and requires general anesthesia to administer. Selective embolization of vesicle arteries by interventional radiology has been used with success in patients with severe intractable bleeding that was refractory to most other treatments (
35). Hyperbaric oxygen has been used for hemorrhagic cystitis with an observed 82% response rate. However, as many as 60 treatments may be needed before the desired effect is seen and this therapy is not widely available at most institutions (
39). In severe cases of hematuria originating from the bladder, a cystectomy may be necessary to stop bleeding.
Radiation cystitis is seen in as many as 8% of patients who have received radiation therapy for prostate cancer.
The management of radiation cystitis may involve many of the treatments discussed above, as it is often recurrent and refractory. Similarly, treatment with chemotherapeutic agents such as cyclophosphamide and iphosphamide may result in hemorrhagic cystitis, a problem that may be avoided if pretreated with Mesna during chemotherapy. Careful surveillance for the presence of malignancy should be done in these patients as the bleeding may represent a secondary malignancy. Treatment of radiation cystitis and hemorrhagic cystitis secondary to chemotherapeutic agents should follow the previously described guidelines for the treatment of hematuria, being as conservative as possible yet aggressive enough to prevent clot retention and further discomfort. Hyperbaric treatment of recurrent radiation cystitis works by increasing oxygenation of the bladder mucosa and may also cause vasoconstriction, resulting in decreased hematuria and healing (
39).


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access




