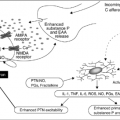
Urologic Issues in Palliative Care
David A. Kunkle
Steven J. Hirshberg
Richard E. Greenberg
The management of patients with a progressive medical disease should permit them to live the remainder of their lives to their fullest potential, maximizing both the quality and quantity of life. The development of complications related to the genitourinary system is not uncommon in these patients. Although some of these problems may merely be considered minor annoyances, others are quite serious and can potentially undermine a patient’s quality of life. The most serious complications may, in fact, reduce life expectancy.
Commonly, classification of genitourinary problems differentiates the urinary tract into upper and lower systems. The upper urinary tract refers to those organs proximal to the ureterovesical junction. The lower urinary tract pertains to the bladder, prostate, and urethra. This chapter discusses the diagnosis and management of both the upper and the lower urinary tract pathology. Specifically, the matters of irritative voiding symptoms, bladder outlet obstruction, upper urinary tract obstruction, hematuria, and priapism are addressed with regard to the palliative care patient.
Irritative Voiding Symptoms
The complex of irritative voiding symptoms refers to the symptoms of urinary frequency, urgency, and dysuria. These symptoms are common in patients seeking urologic evaluation. Rarely, they are the first indication of a severe underlying problem. In addition to symptomatic relief, the management must focus on identifying and treating the underlying disorder (Table 32.1). Investigations may be warranted and modified on the basis of clinical presentation and patient prognosis.
Infection
Urinary tract infection is one of the most common conditions treated by physicians. Typical signs of lower urinary tract infection (simple cystitis) include urinary frequency, urgency, dysuria, foul-smelling urine, hematuria, and suprapubic tenderness. Many cases of urinary tract infection in hospitalized or hospice patients are iatrogenic, secondary to urinary tract manipulation (most often by urethral catheters). When evaluating a patient who presents with acute onset of new irritative voiding symptoms, urinary tract infection should be among the first diagnoses considered. A urinalysis, including both dipstick and microscopic analysis, should be performed before starting empiric antibiotic therapy. Those patients who are either hospitalized, institutionalized, or who have recently been discharged from such a facility should also have a urine culture and sensitivity performed. The potential virulence of the bacterial flora associated with these facilities justifies early culture. Likewise, a patient with recent urinary tract instrumentation, including urethral catheterization, who develops signs of urinary tract infection should also have a urine culture done at initial evaluation.
When obtaining urine specimens from patients who appear to have failed the appropriate antibiotic therapy, it is particularly important to ensure proper collection of the specimen. This especially holds true for debilitated patients and those with significant functional impairments who may not be able to properly collect a clean-catch specimen. If the patient is unable to appropriately collect urine for urinalysis or urine culture, a catheterized specimen may be necessary before continuing or modifying treatment. Additionally, when specimens are collected from patients with either an indwelling catheter or condom catheter, one must take care in interpreting the results. Urine from these patients is almost always colonized with bacteria, and, consequently, the finding of bacteriuria alone does not necessarily indicate active urinary tract infection, although a negative culture under these circumstances is often considered adequate to rule out infection. In the setting of chronic indwelling catheter with urinary bacterial colonization, the decision to treat with antimicrobials may, in part, be determined by the presence or absence of symptoms.
Patients who develop recurrent or relapsing symptomatic infections should undergo further evaluation to rule out structural abnormalities and urinary obstruction. Stasis of urine flow at any level of the urinary tract predisposes an individual to urinary infection by creating a favorable environment for bacterial proliferation. Urinary obstruction may occur in the setting of anatomic abnormalities, obstructing stone or neoplasm, or bladder outlet obstruction. Additional factors contributing to urinary tract infection include diabetes mellitus and immunosuppression as a result of cancer or its therapies. Other patients at particular risk for urinary tract infection include those on chronic steroid therapy and those infected with the human immunodeficiency virus.
Initial evaluation of patients with recurrent urinary tract infections should include measurement of a postvoid bladder residual to rule out the presence of a large volume of residual urine. Although there is no absolute volume of urine to be considered abnormal following urination, an excess of approximately 150–200 mL should prompt therapy to improve bladder emptying. This can be determined by catheterizing a patient once he has voided to completion. As an alternative, the postvoid residual may be measured by ultrasound imaging, which should include measurements of bladder volume both before and after voiding. A newer option for assessment of residual urine is the Bladder Scan device (Diagnostic Ultrasound, Bothell, WA), a portable ultrasound unit, which can be used at the bedside shortly following urination. Additional studies may include renal ultrasound to exclude hydronephrosis, and intravenous pyelogram (IVP) or noncontrast computed tomography (CT) scan evaluation for calculi, hydronephrosis, or diverticuli. Urine culture and sensitivity are considered mandatory when infections do not resolve with empiric antibiotic therapy.
Table 32.1 Irritative Voiding Symptoms | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Tumor-Related Symptoms
Although significantly less common than urinary tract infection, tumor invasion in the bladder is capable of causing irritative voiding symptoms. Tumor invasion may originate from within the bladder, such as in the setting of urothelial carcinoma, or extravesically from within the pelvis. A genitourinary tumor that commonly presents with irritative voiding symptoms is carcinoma insitu of the bladder. Although carcinoma insitu may be completely asymptomatic, it more commonly presents with irritative voiding symptoms. On the other hand, low-grade papillary lesions of the bladder or upper tracts usually have insidious onset and may be completely asymptomatic. Common malignancies originating outside of the bladder include tumors of the ovary, cervix, uterus, rectum, prostate, and colon.
The distinction between tumor invasion and urinary tract infection can usually be made on the basis of the history and several tests. Although the symptomology may be quite similar, direct tumor invasion is more likely to have an insidious onset and also likely to be associated with gross hematuria. All patients with irritative voiding symptoms should undergo a detailed urinalysis (dipstick and microscopic analysis of urine sediment). If a urinary tract infection is excluded by urinalysis and urine culture (when indicated), other etiologies should be considered. The differential diagnosis for noninfectious causes of bladder irritability includes bladder or ureteral calculi (secondary to irritation caused by a calculus in the intramural portion of the distal ureter), foreign bodies, and tumors. Like urinary tract infection, these diseases may produce pyuria on routine urinalysis. However, unless there is concurrent urinary tract infection, urine should be sterile when examined in the setting of these noninfectious etiologies. One notable exception is in the case of sterile pyuria associated with genitourinary tuberculosis.
Any patient with new-onset irritative voiding symptoms and sterile urine should have a voided urinary cytology. Although not necessarily diagnostic of a urothelial tumor, a positive urine cytology is particularly helpful in diagnosing carcinoma insitu of the bladder. However, a negative cytology does not exclude malignancy because of its relatively low sensitivity for detecting low-grade superficial papillary transitional cell carcinomas of the bladder or upper tracts. Overall, urinary cytology has been reported to have a sensitivity of 38.0% and a specificity of 98.3% with improved sensitivity for higher-grade malignancy (1). Other recent advances in the early diagnosis of carcinoma insitu include various urinary markers (e.g., nuclear matrix protein-22, hyaluronic acid-hyaluronidase, BTA-Stat, urinary bladder cancer antigen), which are still under investigation as to their clinical usefulness.
Patients who develop irritative voiding symptoms associated with microscopic or gross hematuria with negative urine cultures also deserve additional evaluation. The accepted evaluation for these patients includes evaluation for upper and lower urinary tract pathology. Traditionally this has included cystoscopy, urine cytologic evaluation, and intravenous urography. In patients with an advanced medical illness, in whom diagnosis of urothelial cancer may not change the overall management, this algorithm may be modified. With the development of fiber optics and flexible cystoscopes, office or bedside cystoscopy may be performed with minimal discomfort. Renal and bladder ultrasound, which can now be performed with relative ease, can detect solid tumors of the kidney or large tumors in the bladder; it would be unlikely, however, to diagnose a small tumor of the urothelium. Additionally, newer techniques in CT scan, with spiral scans and three-dimensional reconstruction, enable detailed evaluation of the entire urinary system with minimal morbidity.
The initial treatment of a tumor that directly invades the bladder wall and causes irritative voiding symptoms should include transurethral resection of the bladder tumor (TURBT). Depending on the extent of tumor, this may be carried out on an outpatient basis, although some form of anesthesia, usually spinal or general, is required. Transurethral resection entails using a special cystoscope (resectoscope) with a cutting “loop.”
By the application of an electrical current, the loop essentially cuts through bladder tissue. This loop is used to resect the abnormal urothelium and the underlying layers (submucosa and muscularis) of the bladder. The diagnostic value of TURBT further supports its acceptance as the procedure of choice for the initial evaluation of all bladder tumors. If a full thickness resection is performed, the depth of tumor invasion and the degree of differentiation can be determined with a single procedure.
By the application of an electrical current, the loop essentially cuts through bladder tissue. This loop is used to resect the abnormal urothelium and the underlying layers (submucosa and muscularis) of the bladder. The diagnostic value of TURBT further supports its acceptance as the procedure of choice for the initial evaluation of all bladder tumors. If a full thickness resection is performed, the depth of tumor invasion and the degree of differentiation can be determined with a single procedure.
Patients who persist with disabling irritative voiding symptoms after TURBT may be treated symptomatically. Anticholinergic drugs such as oxybutynin, tolterodine, and solifenacin are commonly used to treat symptoms of urinary frequency, urgency, and nocturia. These drugs may be started at low doses and then titrated based upon their affect. Caution must be used in those patients who also have a component of bladder outlet obstruction or bowel obstruction because their anticholinergic action may aggravate urinary retention and gastrointestinal dysmotility. A careful observation of voiding and bowel elimination patterns during treatment is required in these patients. At times, a fine balance between irritative voiding symptoms and urinary retention must be obtained. In severe cases, anticholinergic therapy can be used to purposely induce retention, allowing the patient to be effectively managed with intermittent catheterization. In addition, there is a relative contraindication to the use of anticholinergic therapy in patients with closed angle glaucoma. However, if patients maintain routine pharmacologic therapy for this condition, there should be little chance of clinical deterioration (2).
Urinary analgesic drugs, the most well known of which is phenazopyridine (Pyridium), may offer some symptomatic benefit to patients with irritative voiding symptoms. It is converted from its inactive to its active form within the urinary tract. At times, a combination of this treatment and an anticholinergic medication may be particularly helpful in relieving symptoms.
Radiation Cystitis
It is not uncommon for patients to develop irritative voiding symptoms after external beam or interstitial radiation therapy to the pelvis. Radiation therapy is often used to treat genitourinary, gynecologic, and gastrointestinal tumors. Acute radiation cystitis after external beam radiation is characterized by dysuria, frequency of urination, nocturia, and rarely, hematuria. Dean and Lytton (3) evaluated patients after pelvic irradiation and found that 21% reported urologic symptoms. However, in only 2.5% were these truly related to the radiation itself; in most cases, the symptoms were attributable to recurrent or persistent tumor. Newer methods of delivering external beam radiation therapy such as intensity modulated radiation therapy (IMRT) offer the hope of increasing treatment doses while minimizing side effects.
Symptoms usually begin to develop after exposure to 3000 cGy, and there is an increased incidence of cystitis in those patients receiving >6000–6500 cGy. Patients who experience persistent symptoms after completion of radiation fall into the category of chronic radiation cystitis. A history of open bladder surgery, in the subset of patients who receive higher doses of radiation, appears to be an independent risk factor for the development of chronic radiation cystitis.
The associated symptom complex frequently determines measures used to treat acute radiation cystitis. To address symptoms, pharmacotherapy with phenazopyridine or anticholinergic medications, either alone or in combination, are frequently administered. However, those patients who are refractory to these symptomatic treatments can become debilitated and experience a marked decline in quality of life due to severe urinary frequency, urgency, dysuria, nocturia, and at times, urge incontinence. Approaches to treating patients with intractable symptoms are limited to procedures that divert the urine stream. Occasionally, diversion of the urine with a urethral catheter improves symptoms, although frequently the bladder discomfort is actually exacerbated by catheter irritation. Alternatives to catheter drainage, which have been used with limited success, include small suprapubic catheters and bilateral percutaneous nephrostomy diversion.
Invasive approaches aimed at diverting the urine or enlarging the bladder require a major abdominal surgery. A potential deterrent to any surgery involving the bladder in patients with radiation cystitis is the damaging effects of the radiation itself. Risks of peri- or postoperative complications for augmentation cystoplasty or urinary diversion are dramatically increased in patients with irradiated bowel or bladder due to underlying radiation-induced vasculitis.
Augmentation cystoplasty involves enlarging the bladder by surgically incorporating a patch of small bowel, colon, or stomach at the dome of the bladder. Urinary diversion requires constructing either a urostomy with external appliance or a continent diversion by means of a catheterizable pouch or orthotopic neobladder. Similarly, these procedures require the use of segments of small bowel or large bowel, both of which may have been irradiated. If palliative urinary diversion is entertained, the bladder itself does not necessarily need to be removed. In fact, cystectomy should probably be avoided because of the increased surgical risks after radiation therapy. Although surgical diversion and bladder augmentation are considered in this discussion for the sake of completeness, few patients within the palliative care population are actually considered as candidates for this intervention because of the associated surgical risks and the time required for convalescence.
Chemical Cystitis
Several agents that are administered intravesically for the treatment of either superficial or multifocal transitional cell carcinoma of the bladder or carcinoma insitu can be potentially toxic and irritating. All of these agents have the potential to cause a chemical cystitis to varying degrees.
The most common and the most effective medication used for intravesical treatment of noninvasive bladder carcinoma is bacillus Calmette-Guérin (BCG). A standard course of BCG therapy involves weekly bladder instillations for 6 weeks. Depending on the clinical response and the physician’s preference, maintenance therapy may continue after the initial 6-week regimen. Symptoms of urinary frequency, dysuria, and hematuria may develop after two or three instillations and last for approximately 2 days after each treatment. These symptoms are an expected consequence of the immune stimulation and inflammatory reaction that are thought to be essential components of the tumor-suppressive action of BCG (4). Lamm et al. (5) reviewed the complications of BCG therapy in 1278 patients and found that 91% developed dysuria, 90% had urinary frequency, and 43% reported hematuria. These symptoms seemed to increase with both the duration and frequency of treatments. Although BCG is available in several strains, the local irritative symptoms were not limited to any particular strain.
In patients undergoing a 6-week course of therapy, symptoms can usually be well controlled with the combination of phenazopyridine and anticholinergic medications. Although there have not been any randomized or controlled studies of these medications, several investigators with over 2600 patients support their routine use for symptomatic relief (6). For those patients refractory to this regimen, or in those patients who continue with maintenance therapy, treatment with isoniazid, diphenhydramine, acetaminophen, and nonsteroidal anti-inflammatory medications may be helpful. Treatment is usually continued for the duration of symptoms and may be
given prophylactically for 3 days starting on the morning of BCG administration. Because all these agents have a different mechanism of action, they are frequently used in combinations, and doses are titrated until a maximal effect is obtained. A rare complication of treatment with BCG is the development of a nonfunctional contracted bladder, which occurs in approximately 0.2% of cases. Because contracted bladders do not tend to develop in patients with severe irritative symptoms who were treated with prophylactic isoniazid, early treatment with antituberculous medication may be helpful in preventing this disabling complication.
given prophylactically for 3 days starting on the morning of BCG administration. Because all these agents have a different mechanism of action, they are frequently used in combinations, and doses are titrated until a maximal effect is obtained. A rare complication of treatment with BCG is the development of a nonfunctional contracted bladder, which occurs in approximately 0.2% of cases. Because contracted bladders do not tend to develop in patients with severe irritative symptoms who were treated with prophylactic isoniazid, early treatment with antituberculous medication may be helpful in preventing this disabling complication.
Local irritative side effects are seen somewhat less frequently with other intravesical chemotherapeutic regimens. Agents used for intravesical treatment of bladder cancer include triethylenethiophosphoramide (thiotepa), etoglucid (epodyl), mitomycin C, doxorubicin, and interferon-α. Mitomycin C has been associated with chemical cystitis in 10–15% of patients and, in rare cases, can lead to a contracted bladder. Doxorubicin has also been associated with chemical cystitis and defunctionalized bladders. Treatment of cystitis due to these agents is similar to that due to BCG except that antituberculous medications have no effect on the irritative symptoms caused by these agents.
Systemic administration of certain cytotoxic drugs can also lead to irritative symptoms. These symptoms are commonly associated with cyclophosphamide and ifosfamide (oxazaphosphorine alkylating agents), busulfan (1, 4-dimethanesulfonayoxybutane), and methenamine mandelate (7). Such symptoms may be avoided through the concomitant administration of mesna during chemotherapy. Mesna is a chelating agent that binds acrolein, a toxic by-product of phosphamides, thereby decreasing its toxic effects.
In rare cases, irritative voiding symptoms can be refractory to conservative therapies. If the suffering from unrelieved symptoms is severe, urinary diversion may be contemplated. The standard form of urinary diversion with the lowest risk of short-term morbidity is the ileal conduit (see section on Radiation Cystitis). This procedure requires general anesthesia, major intra-abdominal surgery, and a minimum 5- to 7-day postoperative recovery. Although it is an alternative for those patients who have experienced a marked loss of independence and quality of life, the risks associated with the procedure must be weighed against the possible benefits.
Table 32.2 Management Options for Obstructive Uropathy | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Outlet Obstruction
One of the most common problems of the urinary tract in men is bladder outlet obstruction, which can result from either benign or malignant processes. In men, the most common organ to impede bladder emptying is the prostate gland. Bladder outlet obstruction may also occur as a direct consequence of tumor extension or other pathology arising from the rectum or urethra. In addition, a lesion of the ovary, cervix, or uterus may be the cause in women. The ultimate consequence to the patient with obstructive uropathy, without intervention, is renal failure secondary to chronic urinary retention. This condition progresses more rapidly if there is concomitant urinary infection, a situation not uncommon in patients who fail to adequately empty their bladder.
A careful history may disclose the lesion responsible for urinary retention. Physiologically, failure to empty the bladder indicates failure to generate adequate detrusor pressure to overcome urethral resistance. This may result from primary detrusor failure (e.g., associated with diabetes mellitus or sacral plexus injury) or from bladder outlet obstruction at or distal to the bladder neck. A detailed urologic history relating symptoms before the episode of urinary retention may clarify the underlying problem. Patients who complain of urinary frequency, urgency, nocturia, and a slow urinary stream frequently have obstruction as the underlying etiology of their urinary retention. In contrast, patients who report a slow stream, impaired bladder sensation, increasing intervals between voids, and decreased urgency are more likely to have primary detrusor failure. Because the management of these two disorders differs significantly, it is obviously critical to make this distinction before initiating therapy (Table 32.2).
Nonsurgical Treatment
Whereas the initial management of urinary retention secondary to benign prostatic hyperplasia (BPH) may warrant a period of catheter drainage and an empiric trial of α-antagonist medications (terazosin, doxazosin, alfuzosin, or tamsulosin), bladder outlet obstruction secondary to malignant disease is less likely to respond to this approach. Deconditioning and immobility are additional complicating factors in the terminally ill; debilitation may decrease the likelihood of
recovery of adequate bladder detrusor function. Those patients with BPH who fail initial conservative measures (α-blockers and bladder rest) after an adequate time period (e.g., 1 to 2 weeks) may be candidates for alternative measures. These options, which are all invasive to an extent, include chronic urethral or suprapubic bladder drainage, intermittent catheterization, and surgical procedures such as urethral stenting, urinary diversion, or surgical resection of the obstructing tissue. In patients with malignant disease, these approaches should be considered earlier in the course of treatment of the patient.
recovery of adequate bladder detrusor function. Those patients with BPH who fail initial conservative measures (α-blockers and bladder rest) after an adequate time period (e.g., 1 to 2 weeks) may be candidates for alternative measures. These options, which are all invasive to an extent, include chronic urethral or suprapubic bladder drainage, intermittent catheterization, and surgical procedures such as urethral stenting, urinary diversion, or surgical resection of the obstructing tissue. In patients with malignant disease, these approaches should be considered earlier in the course of treatment of the patient.
Hormonal Manipulation
A nonsurgical approach to patients with obstruction due to prostate carcinoma involves hormonal manipulation. This modality should be especially considered for those patients not previously treated in this fashion. Up to 72% of patients with advanced prostate cancer may have symptoms of bladder outlet obstruction (8). Surya and Provet (9) suggest that androgen deprivation may be preferable to surgery as the initial mode of therapy in this group of patients. Androgen deprivation may be accomplished by means of surgical bilateral orchiectomy or “medical orchiectomy” with luteinizing hormone releasing hormone (LHRH) analogs (leuprolide or goserelin) or diethylstilbestrol. In those patients with advanced disease who are to be initiated on LHRH analogs, a recent bone scan should be available to rule out significant occult bony disease in the vertebral column. After initiation of treatment with LHRH agonists, a luteinizing hormone and consequent testosterone surge may occur within the first 2 weeks of therapy; therefore patients with significant vertebral metastases, whether occult or overt, are at significant risk for spinal cord compression. For similar reasons, patients with bone pain may have increased analgesic needs shortly after beginning treatment with LHRH analogs. To reduce the potential for these adverse events, the authors recommend that all patients be started on antiandrogen therapy (flutamide, bicalutamide, nilutamide) before initiation of LHRH agonist therapy to avoid this testosterone flare phenomenon. At a minimum, patients with documented bony disease in the cervical, thoracic, or lumbar axial skeleton should be started on an antiandrogen 7 days before commencing therapy with LHRH analogs. Patients who fail to void 2 to 3 weeks after reduction of serum testosterone to castrate levels usually require surgical intervention or prolonged catheter drainage. It is notable that, in patients with advanced prostate cancer receiving androgen deprivation therapy, the development of obstructive uropathy has been shown to result in significantly reduced survival (10).
Clean Intermittent Catheterization
For the medically ill patient who does not wish to undergo a surgical procedure or who is deemed medically unfit to tolerate surgery, clean intermittent catheterization (CIC) and chronic indwelling catheterization represent common options for adequate bladder decompression. These options are best suited for those with urinary retention secondary to detrusor failure as opposed to outlet obstruction. CIC has been the nonsurgical treatment of choice to empty the bladder ever since Lapides introduced the concept in the late 1960s (11). CIC enables a motivated patient to avoid a chronic indwelling urethral catheter, thereby decreasing the attendant risks of infection, stricture, epididymitis, and symptoms associated with a defunctionalized bladder. Once a patient is placed on an adequate CIC schedule, bladder residuals must be monitored so that upper tract deterioration due to storing urine at high pressures is avoided. Common findings on routine urinalysis in those patients on CIC include pyuria and bacteriuria. Those patients with systemic evidence of infection (i.e., fever, flank pain, and leukocytosis) should receive appropriate antimicrobial treatment. However, in the absence of these symptoms, the use of broad-spectrum antibiotic therapy in an attempt to sterilize the urine should be avoided because of the potential to develop resistant bacterial species. In patients with recurrent urosepsis, however, chronic antibiotic prophylaxis using low-dose antibiotics or urinary antiseptic drug regimens may be considered. Sterile intermittent catheterization may also alleviate problems of recurrent infections in those patients with clinical symptoms. Other issues that must be addressed before initiating CIC, especially in chronically ill patients, are sufficient manual dexterity and an adequate urethral channel to perform catheterization.
Indwelling Catheters
Although the use of indwelling catheters is usually limited to acute urinary retention, the benefits and risks of chronic catheterization in the medically ill patient must be carefully weighed. Frequently, a permanent urethral catheter is the most appropriate method of management in the patient with a very short life expectancy. Because these catheters can be changed at relatively long intervals, they may also be the modality of choice for patients who are technically difficult to catheterize or who are unable to catheterize themselves because of functional impairments. Urethral catheters are relatively simple devices, but may cause significant discomfort in patients who experience bladder spasms. These patients may leak urine around the catheter and experience intense suprapubic discomfort. Treatment for this predicament may include oral anticholinergic medications or suppositories containing belladonna and opium. Other problems with long-term catheterization include obstruction of urine drainage secondary to calcification of the catheter itself, calcification of the catheter balloon, urethral stricture, urethritis, epididymitis, urosepsis, and urethral erosion. Although placement of a suprapubic catheter avoids urethral trauma and irritation and decreases the risk of urosepsis, significant complications may still occur.
Surgical Treatment
Transurethral Resection of the Prostate
During a transurethral resection of the prostate (TURP), benign or malignant prostatic tissue is resected using an electrocautery loop. Although the procedure itself is usually limited to 1 hour, potential exists for the development of significant complications, especially in patients with underlying cardiac disease. TURP has the potential to create large fluid shifts by the absorption of irrigation fluid through prostatic venous channels during resection. In a patient with underlying cardiac disease, this may cause congestive heart failure in an otherwise compensated patient. Other inherent risks associated with this procedure include bleeding, which has the potential to be significant during resection of a large gland; urinary tract infection; urethral stricture; dilutional hyponatremia; bladder perforation; erectile dysfunction; postoperative hydronephrosis; and urinary incontinence. Newer potassium-titanyl-phosphate (KPT) and holmium lasers are now available for vaporization of the prostate due to BPH. These devices offer decreased surgical morbidity and decreased postoperative catheter times when compared to standard TURP.
In patients who fail other treatments, TURP remains the gold standard for removal of obstructing prostatic tissue. Several newer techniques, however, have been developed for resecting, evaporating, or coagulating the obstructing prostate. These newer techniques include electrosurgical vaporization of the prostate, transurethral needle ablation, microwave
therapy, and high-frequency radio wave ablation. Several of these “minimally invasive” devices are now approved by U.S. Food and Drug Administration (FDA) for treatment of benign disease but no major studies have examined their use in patients with malignant disease of the prostate. Overall, these alternative methods seem to promise a rapid relief of symptoms with relatively low morbidity. However, long-term data to support sustained results for these techniques is still being collected. The role of these treatments for palliation has yet to be defined.
therapy, and high-frequency radio wave ablation. Several of these “minimally invasive” devices are now approved by U.S. Food and Drug Administration (FDA) for treatment of benign disease but no major studies have examined their use in patients with malignant disease of the prostate. Overall, these alternative methods seem to promise a rapid relief of symptoms with relatively low morbidity. However, long-term data to support sustained results for these techniques is still being collected. The role of these treatments for palliation has yet to be defined.
Palliative Transurethral Resection of the Prostate
Bladder outlet obstruction may occur because of prostate cancer, and it has been estimated that 25–35% of patients on a watchful waiting treatment plan for prostate cancer may ultimately require TURP (12). An alternative to a standard TURP, in which all of the prostatic tissue is removed out to the surgical capsule, is the “channel” TURP performed for palliation. Channel TURP is defined as a TURP performed to alleviate obstructive voiding symptoms in the patient with advanced or previously treated cancer. It may also refer to a TURP performed to remove obstructing prostatic tissue without necessarily resecting all of the tissue to the surgical capsule. Advantages of channel TURP as against traditional TURP arise from performing a more limited operation with less associated operative morbidity. The risk of urinary incontinence is of particular concern during resection of malignant prostatic tissue, which may cause alteration of normal anatomic landmarks and may directly invade the external urethral sphincter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree