Despite being the most common sarcoma of the gastrointestinal tract, gastrointestinal stromal tumor (GIST) has been widely recognized as a unique entity for just over a decade. The advent of tyrosine kinase inhibitors has revolutionized the diagnosis and treatment of GIST. Although surgery remains the only chance for cure, multimodal treatment that includes molecular therapy continues to develop. Optimal management of GIST requires careful radiographic, pathologic, medical, and surgical care, emphasizing the need for a multidisciplinary approach. This review highlights recent developments in the management of GIST.
Epidemiology
Gastrointestinal stromal tumor (GIST) is the most frequently encountered mesenchymal tumor of the gastrointestinal tract. Although the annual incidence in the United States is reported to be approximately 5000 cases per year, the true incidence is difficult to determine because it was only recently classified as a separate entity from leiomyoma, leiomyosarcoma, and leiomyoblastoma. Because of increased awareness and improved histopathologic detection, the incidence of GIST seems to be increasing. GISTs affect men and women equally, except for pediatric GISTs, which occur predominantly in girls. Although GISTs have been reported in all age groups, including newborns, they are uncommon in patients less than 30 years old. Most patients diagnosed with GIST are between 40 and 80 years old, with a median age at diagnosis of 60 years.
The most commonly encountered GIST is the sporadic form. Familial GISTs occur and result from a germline mutation in either the KIT or platelet-derived growth factor receptor α ( PDGFRα) proto-oncogenes. GIST can also occur in patients with neurofibromatosis type 1 (NF1) and in young women as part of a syndrome that includes paragangliomas, pulmonary chondromas, and gastric GISTs (ie, the Carney triad).
Clinical presentation and diagnosis
GISTs can cause a variety of symptoms ranging from vague abdominal pain to peritonitis as a result of tumor rupture and intraperitoneal bleeding. Other modes of presentation include abdominal fullness, early satiety, weakness, and fatigue secondary to anemia from occult gastrointestinal bleeding. Bowel obstruction is rare. Small GISTs (<3 cm) are often detected incidentally on computed tomography (CT) scans, endoscopy, or at the time of laparotomy for other indications. Lesions discovered incidentally and at autopsy have been shown to measure 2.7 cm and 3.4 cm, respectively. Median tumor size at presentation in symptomatic patients is 5 cm.
GISTs can occur anywhere in the gastrointestinal tract from the esophagus to the rectum. The stomach represents the most common site (60%), followed by the small bowel (30%), rectum (∼5%), and esophagus (∼5%). The clinical course of GIST can range from benign to malignant. Up to 50% of patients present with metastatic disease at the time of diagnosis, with the liver and peritoneum being the 2 most common sites of extraintestinal spread. Occasionally, patients present with primary GISTs of the omentum, mesentery, or pancreas.
Because of the wide range of symptoms and its rarity, the diagnosis of GIST requires a high index of suspicion. The primary mode of diagnosis and assessment of the extent of disease is by contrast-enhanced CT scan of the abdomen and pelvis. Characteristic findings on CT scan include an enhancing, exophytic mass in close association with the stomach or bowel wall. Like other sarcomas, GISTs tend to displace rather than invade adjacent structures. Occasionally, larger GISTs (>10 cm) can show heterogeneity on CT, which usually signifies hemorrhage or occasionally necrosis within the tumor. Magnetic resonance imaging can be useful in cases of rectal GIST. Although positron emission tomography (PET) is not used to diagnose GIST, it can be helpful in assessing the response to tyrosine kinase therapy. PET can also be useful in patients with metastatic disease who are being considered for surgery or those on second-line agents after failure of imatinib, in whom mixed responses may occur. On endoscopic evaluation, GIST appears as a submucosal mass. Although endoscopic or percutaneous biopsy is recommended in cases in which neoadjuvant therapy or metastasis is suspected, the role of routine biopsy of isolated lesions is controversial. Endoscopic-guided fine needle aspiration has been shown to be ∼80% sensitive for diagnosing GIST. Because GISTS tend to be soft and friable, biopsy carries the risk of tumor rupture, bleeding, and dissemination.
Clinical presentation and diagnosis
GISTs can cause a variety of symptoms ranging from vague abdominal pain to peritonitis as a result of tumor rupture and intraperitoneal bleeding. Other modes of presentation include abdominal fullness, early satiety, weakness, and fatigue secondary to anemia from occult gastrointestinal bleeding. Bowel obstruction is rare. Small GISTs (<3 cm) are often detected incidentally on computed tomography (CT) scans, endoscopy, or at the time of laparotomy for other indications. Lesions discovered incidentally and at autopsy have been shown to measure 2.7 cm and 3.4 cm, respectively. Median tumor size at presentation in symptomatic patients is 5 cm.
GISTs can occur anywhere in the gastrointestinal tract from the esophagus to the rectum. The stomach represents the most common site (60%), followed by the small bowel (30%), rectum (∼5%), and esophagus (∼5%). The clinical course of GIST can range from benign to malignant. Up to 50% of patients present with metastatic disease at the time of diagnosis, with the liver and peritoneum being the 2 most common sites of extraintestinal spread. Occasionally, patients present with primary GISTs of the omentum, mesentery, or pancreas.
Because of the wide range of symptoms and its rarity, the diagnosis of GIST requires a high index of suspicion. The primary mode of diagnosis and assessment of the extent of disease is by contrast-enhanced CT scan of the abdomen and pelvis. Characteristic findings on CT scan include an enhancing, exophytic mass in close association with the stomach or bowel wall. Like other sarcomas, GISTs tend to displace rather than invade adjacent structures. Occasionally, larger GISTs (>10 cm) can show heterogeneity on CT, which usually signifies hemorrhage or occasionally necrosis within the tumor. Magnetic resonance imaging can be useful in cases of rectal GIST. Although positron emission tomography (PET) is not used to diagnose GIST, it can be helpful in assessing the response to tyrosine kinase therapy. PET can also be useful in patients with metastatic disease who are being considered for surgery or those on second-line agents after failure of imatinib, in whom mixed responses may occur. On endoscopic evaluation, GIST appears as a submucosal mass. Although endoscopic or percutaneous biopsy is recommended in cases in which neoadjuvant therapy or metastasis is suspected, the role of routine biopsy of isolated lesions is controversial. Endoscopic-guided fine needle aspiration has been shown to be ∼80% sensitive for diagnosing GIST. Because GISTS tend to be soft and friable, biopsy carries the risk of tumor rupture, bleeding, and dissemination.
Pathologic findings
There are 3 histologic subtypes of GIST. The spindle cell form is the most common (70%) and consists of uniform, intersecting fascicles with eosinophilic cytoplasm. The epithelioid (20%) and the rare mixed type (10%) forms show more rounded cells with nuclear atypia. Approximately 95% of GISTs stain positive for KIT (CD117) by immunohistochemistry (IHC). Epithelioid GISTs tend to have weaker KIT staining than the spindle cell type. Other commonly expressed markers include CD34 (70%), smooth muscle actin (30%), and desmin (<5%). Although immunophenotype is an important component in the diagnosis of GIST, it is not sufficient. Other malignancies that can stain positive for KIT include metastatic melanoma, angiosarcoma, small cell lung cancer, and Ewing sarcoma. The diagnosis of GIST is based on concordance between the morphology and the IHC. In addition, mutation analysis is sometimes required.
GISTs are believed to arise from the interstitial cells of Cajal as a result of a gain of function mutation in the KIT proto-oncogene. KIT mutations can vary and occur in up to 85% of GISTs. The most common sites of KIT mutation include exon 11 (70%) and exon 9 (10%). Other described regions include exons 13, 14, and 17. Recently, ETV1 was shown to be a critical transcription factor in KIT oncogenesis and the development of GISTs. Approximately 10% of patients with GIST instead have a mutation in the PDGFRα proto-oncogene. Approximately 5% to 10% of patients do not carry a mutation in either of the proto-oncogenes described earlier and are classed as having wild-type (WT) GISTs. A subset of these patients have a BRAF mutation. DOG1 (a calcium-dependent chloride channel) is also expressed commonly in GIST and can be useful in establishing the diagnosis.
Risk stratification
Prognosis in GIST is highly variable. The critical determinants of GIST behavior include tumor size, mitotic rate, and location ( Table 1 ). Small tumors (<2 cm) with low mitotic rates (<5 per 50 high power fields [HPF]) show benign behavior, whereas larger tumors (>5 cm) with high mitotic rates (>10 per 50 HPF) are associated with malignant behavior and display higher rates of recurrence after surgical resection. Tumors located in the stomach have favorable outcomes relative to small bowel tumors. Of the 3 determinants of behavior mentioned earlier, mitotic rate is considered the most significant. Small tumors with low mitotic rates have still been shown to display malignant behavior.
| Tumor Parameters | Percentage of Patients with Progressive Disease During Long-term Follow-up and Characterization of Risk for Metastasis | |||||
|---|---|---|---|---|---|---|
| Group | Size (cm) | Mitotic Rate (per 50 HPF) | Gastric GIST s | Jejunal and Ileal GIST s | Duodenal GIST s | Rectal GIST s |
| 1 | ≤2 | ≤5 | 0, none | 0, none | 0, none | 0, none |
| 2 | >2 ≤ 5 | ≤5 | 1.9, very low | 4.3, low | 8.3, low | 8.5, low |
| 3a | >5 ≤ 10 | ≤5 | 3.6, low | 24, moderate | — | — |
| 3b | >10 | ≤5 | 12, moderate | 52, high | 34, high a | 57, high a |
| 4 | ≤2 | >5 | 0 | 50 | — | 54, high |
| 5 | >2 ≤ 5 | >5 | 16, moderate | 73, high | 50, high | 52, high |
| 6a | >5 ≤ 10 | >5 | 55, high | 85, high | — | — |
| 6b | >10 | >5 | 86, high | 90, high | 86, high a | 71, high a |
a Groups 3a and 3b or 6a and 6b are combined in duodenal and rectal GISTs because of the small number of cases.
Gene locus as well as the type of mutation can also affect prognosis. Molecular analysis of the KIT proto-oncogene has revealed that tumors with exon 9 mutations or deletions in exon 11 are more aggressive compared with those harboring either a point mutation or insertion in exon 11. Recurrence after surgery is more common in patients with a deletion mutation in exon 11.
In patients with PDGFRα mutations, location is also important. Exon 18 D842V mutations are resistant to imatinib therapy, whereas those in exon 12 are responsive to imatinib. WT GISTs are associated with imatinib resistance and portend an unfavorable prognosis. Insulinlike growth factor receptor 1 (IGFR1) has been shown to be overexpressed in patients with WT GISTs. In vitro suppression of IGFR1 results in apoptosis of imatinib-sensitive and imatinib-resistant WT GIST cells. Trials to investigate the efficacy of IGFR1 inhibitors in patients with WT GISTs are under way. More recently, a germline mutation in the succinate dehydrogenase ( SDH ) gene was found in 12% of patients with WT GISTs. Defective cellular respiration as a result of SDH mutations in a subset of younger patients with WT GIST is believed to contribute to GIST oncogenesis. Aneuploidy and telomerase expression have both been shown to correlate with worse outcome and the development of metastatic disease.
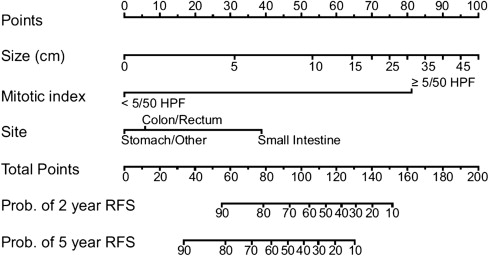
Tumor rupture before or during dissection portends a worse outcome manifested by higher rates of peritoneal recurrence. When examining a specimen, pathologists must consider a slew of prognostic factors that enable them to ultimately categorize GISTs as very low, low, intermediate, or high risk for malignancy. A prognostic nomogram developed at Memorial Sloan-Kettering Cancer Center (MSKCC) that takes into account tumor size, mitotic rate, and location can now be used to assess 2-year and 5-year recurrence-free survival in patients undergoing potentially curative resection of localized primary GIST ( Fig. 1 ). Although the nomogram was developed using 127 patients at MSKCC, it has been validated using 2 patient cohorts from other institutions. The fact that inclusion of tyrosine kinase mutation status failed to improve discriminatory ability may reflect only the limited sample size and number of mutation subtypes.
Treatment
Primary Resectable Disease
Surgery remains the only chance for cure in patients with localized, primary GIST. The goal is to achieve negative microscopic margins with an intact tumor pseudocapsule. Wide margins have not been shown to improve outcomes. Complete resection can usually be accomplished via wedge resection of the stomach or segmental resection of the bowel. Because GISTs spread hematogenously or by local invasion, lymphadenectomy is not routinely required unless adjacent nodes are obviously enlarged. En bloc resection is needed when adjacent organs seem to be involved.
Although there is little disagreement that all tumors larger than 2 cm should be resected, the management of incidentally discovered small GISTs less than 2 cm is controversial. In the absence of high-risk features on endoscopic ultrasonography (EUS) (echogenic foci, ulceration, irregular margins), some have advocated following these lesions with serial imaging or endoscopy. A retrospective analysis looking at the rate of growth of smaller GISTs using EUS found that ∼13% with low-risk features on endoscopy progressed to a point at which they were eventually resected. The usefulness of EUS in the management of small GISTs remains unclear. The frequency of imaging is not well defined, and the need for potentially lifelong surveillance makes this option challenging for some patients and physicians. Although endoscopic resection has been suggested by some, the risk of positive margins, perforation, and tumor spillage make this option generally less desirable. Current National Comprehensive Cancer Network (NCCN) guidelines for the management of gastric GISTs less than 2 cm without high-risk features on EUS include surveillance endoscopy every 6 to 12 months.
The role of laparoscopy in the management of patients with GIST continues to expand. The same principles of complete resection with careful intraoperative handling of tumors apply. Laparoscopic resection of localized gastric GISTs has been studied most extensively thus far. A recently published article from MKSCC studied patients with gastric tumors up to 8 cm. Those undergoing laparoscopic resection had equivalent perioperative and oncologic outcomes compared with case-matched controls undergoing open resection. There was no operative mortality, and 30-day morbidity was similar. Oncologic outcomes were also similar, with no positive microscopic margins and 1 recurrence in each group (median follow-up of 34 months). Nishimura and colleagues reported similar results comparing laparoscopic resection with laparotomy in 67 patients with gastric GISTs ranging from 2 to 10 cm.
Adjuvant Therapy
With surgery alone, recurrence rates approached 50% irrespective of negative margins. Conventional adjuvant therapies such as chemotherapy and radiation have not proved effective in GIST. Response rates of 5% have been reported with chemotherapy, and radiation is seldom used because of the difficulty sparing adjacent healthy tissue. Median survival for patients with GIST treated with cytotoxic chemotherapy is approximately 12 months. Moreover, hepatic artery embolization and intraperitoneal chemotherapy have also resulted in discouraging outcomes.
The approval of imatinib mesylate for the treatment of GIST, both adjuvant and therapeutic, has revolutionized the field. As a specific tyrosine kinase inhibitor (TKI), imatinib has shown efficacy in patients with both KIT and PDGFRα mutations. Imatinib is dosed orally once or twice a day and is generally well tolerated, with rash, diarrhea, and abdominal pain being the most commonly reported side effects.
In a phase II trial led by the American College of Surgeons Oncology Group (ACOSOG), oral imatinib for 12 months after resection in patients with high-risk GISTs was shown to improve recurrence-free survival and increase overall survival compared with historical controls. High risk in this study was defined as a tumor greater than 10 cm, spillage during resection, or more than 5 tumors per patient. In 2009, results from a randomized, placebo-controlled, multicenter phase III trial with 713 patients were reported. Imatinib taken once a day for 1 year after surgery for localized, primary GIST (≥3 cm) was compared with placebo. Recurrence-free survival was significantly higher in the imatinib arm (98%) versus the placebo group (83%) ( Fig. 2 ). Although overall survival was no different, longer follow-up in this patient cohort is needed to definitively determine whether or not adjuvant imatinib can improve overall survival. The cross-over study design may also confound overall survival as an end point. In 2009, the US Food and Drug Administration approved imatinib for use in the adjuvant setting. To define the most effective length of adjuvant imatinib therapy, the results of a recently completed randomized trial comparing 1 year with 3 years of adjuvant imatinib are being finalized. It seems that overall survival is longer with 3 years versus 1 year of adjuvant imatinib. The goal is now to determine which subset of patients with resectable disease truly benefits from adjuvant imatinib. The use of a prognostic nomogram to assess risk of recurrence coupled with mutational analysis may shed some light on this important question.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



