The seventh edition of TNM for Lung Cancer came into effect on the first of January 2011. Containing more than 100,000 cases of lung cancer treated by all modalities of care and from around the world, this is the largest database ever accumulated for this purpose. This edition allows detailed analysis and intensive validation such that the resultant classification aligns stage with prognosis more closely than ever before. There have been changes to certain tumor and metastasis descriptors and the resultant stage groupings. This article describes these changes, provides more in-depth discussion of the changes, and provides much additional information.
Among the many prognostic factors identified in Lung Cancer, the anatomic extent of disease, as described by the tumor-node-metastasis (TNM) classification, has the longest history and remains the most important. Devised by Pierre Denoix, a surgical oncologist at the Institut Gustave-Roussy in Paris and reported in a series of articles between 1943 and 1952, the first international attempt to unify the classification of malignant tumors using this nomenclature was published by the International Union Against Cancer (UICC) in 1968. Thereafter, the initiative passed to the American Joint Committee on Cancer Staging and End Results Reporting (AJC), now the American Joint Committee on Cancer (AJCC), whose Task Force on Lung Cancer, headed by Mountain and colleagues, produced the first data-driven recommendations for change in 1974. Their proposals were derived from a database of 2155 cases of lung cancer, of which 1712 were cases of non–small cell lung cancer (NSCLC), diagnosed at least 4 years before analysis. Practically all of the T descriptors in use today were introduced in that report, including the use of a 3-cm cutoff point for size; the impact on T category of invasion of the chest wall, diaphragm, and mediastinum; the bronchoscopic criteria of T category; and those based on the extent of atelectasis or obstructive pneumonitis. These proposals were incorporated in to the second edition of the UICC TNM Classification of Malignant Tumors published in 1975 and the first publication by the AJC in 1977. Dr Clifton Mountain developed his own database at the MD Anderson Cancer Center in Houston and the reiterative analysis of this developing source informed all future editions of the TNM classification for lung cancer up to and including the sixth edition published in 2002. However, in the intervening years, the process of revision was increasingly considered to be unsatisfactory. The international staging system had been based on data from a single institution, leading to doubts as to whether it was appropriate for global implementation. No external validation had ever been applied, and, even at the last analysis in 1997, the database consisted of slightly more than 5000 cases, too few to validate the large number of descriptors within the TNM classification. The cases were predominately surgical candidates, and TNM staging was increasingly thought to be irrelevant by those treating the bulkier tumors treated by nonsurgical modalities.
In response to these concerns, in 1998 the International Association for the Study of Lung Cancer (IASLC) was convinced that it should develop a role in future revisions and established its Lung Cancer Staging Project. It developed an international database in collaboration with Cancer Research and Biostatistics (CRAB), a not-for-profit organization based in Seattle (OR). Forty-six data sources in more than 20 countries around the globe donated more than 100,000 cases. After an initial sift, 81,495 cases have adequate data to be included in an analysis: 68,463 cases of NSCLC and 13,032 cases of small cell lung cancer (SCLC). The recommendations for the seventh edition of TNM were based on analysis of the NSCLC cases. Of those receiving active therapy, approximately half had undergone surgery as part of their treatment, around one-third had received chemotherapy, one-quarter radiotherapy, and there were many cases treated by bimodality and multimodality treatment. The analysis of this huge number of cases was conducted by CRAB and supervised by several subcommittees tasked with formulating recommendations on the T, N, and M descriptors and the role of TNM in SCLC. All such recommendations were overseen by a validation and methodology subcommittee who established strict criteria for internal and external validation. Once the T, N, and M descriptors had been defined, the resultant TNM stage groups were established using recursive partitioning and amalgamation statistical methods. The recommendations of the IASLC were submitted to the UICC and AJCC and, after internal review, were accepted without change for the seventh edition of TNM for lung cancer enacted in January 2011.
The changes to the T and M descriptors in the 7th edition are shown in Box 1 .
The changes to T and M descriptors in the seventh edition of TNM were:
- 1.
A new cutpoint of 2 cm was created dividing T1 tumors into T1a ≤2 cm and T1b tumors >2 cm but ≤3 cm.
- 2.
A new cutpoint of 5 cm was created dividing T2 tumors into T2a >3 cm but ≤5 cm and T2b tumors >5 cm but ≤7 cm.
- 3.
A new cutpoint of 7 cm was created and tumors >7 cm are now classified as T3, size for the first time becoming a T3 descriptor.
- 4.
Tumors associated with additional tumor nodules in the same lobe as the primary are reclassified as T3.
- 5.
Tumors associated with additional tumor nodules in other ipsilateral lobe(s) are reclassified as T4.
- 6.
Tumors associated with additional tumor nodules in the contralateral lung remain M1 but are reclassified as M1a.
- 7.
Tumors associated with malignant pleural/pericardial effusion or pleural/pericardial nodules are reclassified as M1a.
- 8.
Tumors associated with distant metastases are reclassified as M1b.
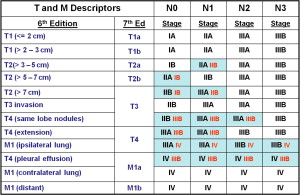
Fig. 1 shows the T and M descriptors as they were in the sixth edition and as they are now classified in the seventh edition, with the resultant changes to the stage groupings.
The full list of T, N, and M descriptors in the seventh edition is given in Box 2 .
T – Primary Tumour
TX Primary tumour cannot be assessed, or tumour proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy
T0 No evidence of primary tumour
Tis Carcinoma in situ
T1 Tumour 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)
T1a Tumour 2 cm or less in greatest dimension a
T1b Tumour more than 2 cm but not more than 3 cm in greatest dimension
T2 Tumour more than 3 cm but not more than 7 cm; or tumour with any of the following features b :
- •
Involves main bronchus, 2 cm or more distal to the carina
- •
Invades visceral pleura
- •
Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung
T2a Tumour more than 3 cm but not more than 5 cm in greatest dimension
T2b Tumour more than 5 cm but not more than 7 cm in greatest dimension
- •
T3 Tumour more than 7 cm or one that directly invades any of the following: chest wall (including superior sulcus tumours), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumour in the main bronchus less than 2 cm distal to the carina a but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumour nodule(s) in the same lobe as the primary.
T4 Tumour of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina; separate tumour nodule(s) in a different ipsilateral lobe to that of the primary.
N–Regional Lymph Nodes
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension
N2 Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s)
N3 Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s)
M–Distant Metastasis
M0 No distant metastasis
M1 Distant metastasis
M1a Separate tumour nodule(s) in a contralateral lobe; tumour with pleural nodules or malignant pleural or pericardial effusion c
M1b Distant metastasis
a The uncommon superficial spreading tumour of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a.
b T2 tumours with these features are classified T2a if 5 cm or less or if size cannot be determined, and T2b if greater than 5 cm but not larger than 7 cms.
c Most pleural (pericardial) effusions with lung cancer are due to tumour. In a few patients, however, multiple microscopical examinations of pleural (pericardial) fluid are negative for tumour, and the fluid is non-bloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumour, the effusion should be excluded as a staging element and the patient should be classified as M0.
The resultant stage groupings in the seventh edition of TNM are presented in Table 1 .
| Occult Carcinoma | TX | N0 | M0 |
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1a, b | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T2b | N0 | M0 |
| T1a, b | N1 | M0 | |
| T2a | N1 | M0 | |
| Stage IIB | T2b | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T1a, b, T2a, b | N2 | M0 |
| T3 | N1, N2 | M0 | |
| T4 | N0, N1 | M0 | |
| Stage IIIB | T4 | N2 | M0 |
| Any T | N3 | M0 | |
| Stage IV | Any T | Any N | M1 |
Additional recommendations
The TNM classification has traditionally applied to all carcinomas of the lung, including SCLC. However, the TNM classification has only been applied to those unusually localized cases of SCLC suitable for surgical treatment, oncologists preferring the simpler, binary division into limited disease and extensive disease. The IASLC Staging Committee, having formulated its recommendations for the seventh edition of TNM from an analysis of the 68,463 cases of NSCLC, studied the usefulness of the TNM classification in the 13,032 cases of SCLC in the database, the largest database of this cell type in the literature. TNM stage details, using the sixth edition of TNM, were available on 8088 patients; 3430 cM0 cases had full clinical TNM data and 4530 were classified as cM1. The analysis of these cases confirmed the value of TNM in the clinical staging of SCLC, a finding that remained relevant for the smaller number of cases suitable for reclassification according to the seventh edition. The SCLC cases in the database contained 349 cases that had undergone complete surgical resection and thus had pathologic TNM staging information, including 218 cases in which there was also cTNM data. Analysis of these cases confirmed the usefulness of TNM in the pathologic classification of SCLC. Therefore, the use of TNM for SCLC cases is strongly encouraged in the seventh edition of TNM , especially for stratification in trials of chemotherapy/radiotherapy in limited disease and for those cases treated by surgery.
Carcinoid tumors were not previously included in the TNM classification. The IASLC database contained 513 cases of carcinoid tumors and was combined with 1619 cases from the Surveillance Epidemiology and End Results (SEER) database for a similar period, of which 1437 had been treated surgically. Analysis of these cases has shown that TNM has prognostic power in this tumor type. As a result, carcinoid tumors of the lung and tracheobronchial tree are now, for the first time, included in the seventh edition of TNM .
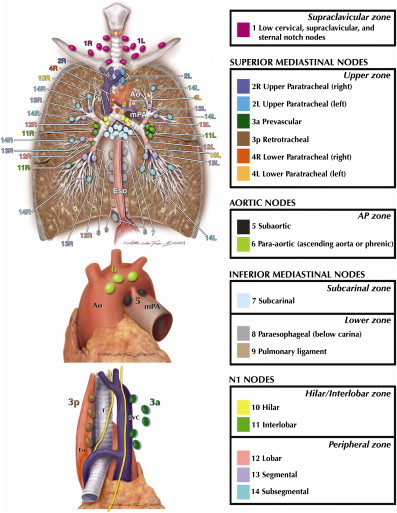
The subcommittee analyzing the N descriptors was hampered by a lack of uniformity in the nomenclature of the regional lymph nodes. The IASLC Staging Project sought international agreement on a new nodal chart that, for the first time, has reconciled the Japanese Naruke and US Mountain/Dressler nodal charts and allowed the development of agreed definitions for the anatomic boundaries of each nodal station. The UICC and AJCC have now recognized this chart and the associated definitions as the “recommended means of describing regional lymph node involvement for lung cancer” ( Fig. 2 ). This development has allowed for the reintroduction of a minimum number of lymph nodes to be removed and examined histologically before assigning a pN category. The seventh edition recommends that “6 nodes/nodal stations should be removed/sampled for histologic examination. These should include 3 nodes/stations from the mediastinum, one of which should be sub-carinal node #7, and 3 nodes/stations from the hilum or other N1 locations.”
Visceral pleural invasion has been a T2 descriptor since the early 1980s. However, there was no agreed definition of this feature. The pathologists within our committee reviewed the available literature and developed a precise definition of visceral pleural invasion as “invasion beyond the elastic layer, including invasion to the visceral pleural surface.” The use of elastic stains is recommended when this feature is not clear on routine histology ( Fig. 3 ).