Update on the Surgical Management of Breast Cancer: Areas of Controversy and Advancement
 ABSTRACT
ABSTRACT
The surgical management of breast cancer has evolved over the past decade and has resulted in several major developments. These include sentinel lymph node biopsy (SLNB), the increased aesthetically enhancing reconstructive techniques in conjunction with breast-conserving surgery and mastectomy, and increased patient preference for preventive procedures. Despite these developments, there are still several controversies in the surgical management of breast cancer. In this review, we discuss the following areas of the surgical treatment of breast cancer which are currently in evolution including criteria and timing of SLNB, intraoperative sentinel lymph node (LN) examination, micrometastatic LN involvement, local surgery for patients presenting with metastatic disease, and oncoplastic considerations in breast cancer.
Keywords: breast cancer, axillary lymph node dissection, sentinel lymph node biopsy, micrometastasis, controversy, skin-sparing mastectomy
 INTRODUCTION
INTRODUCTION
Breast cancer is the most commonly diagnosed female cancer in the United Sates accounting for 27% (192,370) of all new cancer cases and is the second cause of cancer-related death in women (15%) only proceeded by lung cancer (1). Globally, the burden of breast cancer in women as measured by incidence, mortality, and economic costs is increasing, affecting more than one million women in 2002. There is also evidence that this burden is shifting to affect the less developed countries in the world because of their limited screening and diagnostic and treatment resources (2,3). Surgical treatment of breast cancer has evolved over the past decade in parallel with improvements in screening, diagnosis, and treatment. In this review, we have focused on areas for which management is still unclear because of the lack of prospective randomized clinical trials.
Axillary disease burden is currently the best indicator of disease prognosis and guides adjuvant treatment. Prior to the development and institution of sentinel lymph node biopsy (SLNB), all patients with newly diagnosed breast cancer underwent an axillary lymph node dissection (ALND) defined as removal of level I and level II nodes or all lymph nodes (LNs) medial to the pectoralis minor. With improved mammographic screening resulting in the diagnosis of earlier stage breast cancers, an increasing proportion of women were found to have no metastatic disease in their LNs. Women without disease in their LNs are unlikely to receive any therapeutic benefit from an axillary clearance based on the results of NSABP-B04. In this clinical trial, patients were randomized to three arms of axillary treatment: ALND at the time of surgery, radiation therapy to the axilla, or no axillary treatment. This study concluded that removal of occult positive nodes at the time of surgery did not result in a survival benefit (4).
SLNB is a technique developed approximately 15 years ago for staging lymphatic basins in patients with melanoma. It has proven to be an effective and reliable alternative initial staging method for patients with a clinically negative axilla. The reported false-negative rate for patients with breast cancer is in the range of 0% to 8% (5,6). SLNB is associated with fewer complications than ALND. Chronic pain, sensory changes, weakness, and lymphedema are less frequently reported by patients who have had SLNB compared with ALND (7–9). SLNB is based on the finding of hierarchical spread of cancer cells within the axillary LN basin. SLN mapping is performed by the injection of either radioisotope (technicium99 sulfur colloid) or blue dye injection (lymphazurin or methylene blue), or the combination of both into the retroareolar breast tissue or peritumoral. Use of both blue dye and radioactive sulfur colloid was first described by Albertini et al. Other studies comparing the use of single-agent lymphatic mapping with dual agents has demonstrated similar SLN detection rates for single and dual agent use (86% vs 90%). However, there is a significant reduction in the false-negative rate from 11.8% for single agent to 5.8% for dual agents (P < .05). Moreover, the dual injection resulted in an increase in the number of sentinel lymph nodes (SLNs) identified (10,11).
Although it is considered less invasive, SLNB is a surgical procedure that carries its own morbidity. Results from the American College of Surgeons Oncology Group (ACOSOG) Z0010 trial which examined the prognostic significance of metastasis in SLN and bone marrow in patients with clinical T1–2 N0 breast cancer reported complications associated with SLNB. This trial was completed in 2003 and included 5539 patients who experienced the following early complications from SLNB: anaphylaxis (0.1%), wound infection (1%), axillary seroma (7.1%), and hematoma (1.4%). Long-term complications included proximal upper extremity lymphedema (6.9%), axillary paresthesia (8.6%), and decreased upper extremity range of motion (3.8%) (12).
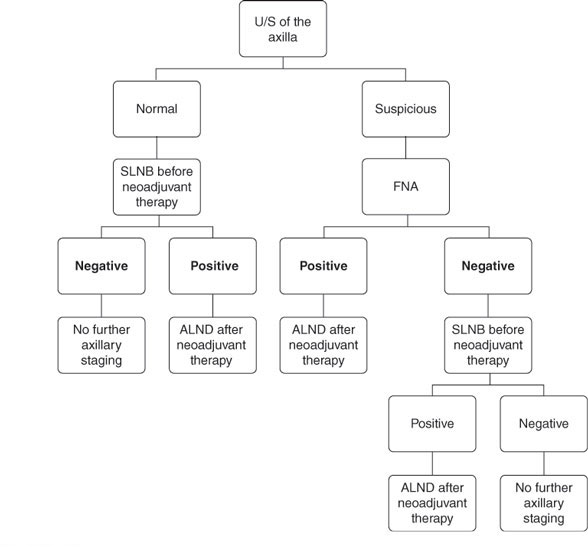
Ultrasound (US)-guided fine-needle aspiration (FNA) of the axilla has been used increasingly for the initial axillary evaluation of patients with early-stage breast cancer and clinically negative axilla (Figure 1) (13,14). LNs which meet the criteria for appearing radiographically abnormal (size greater than 10 mm, absence of fatty hilum, and hypoechoic internal echo with cortical thickening) (15) are assessed for malignant cells by US-guided FNA. In several series, this procedure is reported to have a sensitivity of 75% and a specificity of 100%. This procedure allows the triage of patient with positive LNs directly to ALND without the need for SLNB. Patients who are US or FNA negative proceed to SLNB because of the high false-negative rate with this procedure (13,16).
Currently, the standard of care is completion ALND (cALND) for patients with positive SLN. Recently, this concept has been challenged. Giuliano et al. (17) presented results from the ACOSOG Z0011 trial which randomized patients to cALND or observation for patients with positive SLNB and reported no difference in axillary recurrence between the two groups. Whether this will result in the abandonment of ALND in select populations of patients with positive SLNB remains to be determined.
FIGURE 1
Early-stage invasive breast cancer diagnosis.
 TIMING OF SLNB IN REL ATION TO NEOADJUVANT SYSTEMIC THERAPY
TIMING OF SLNB IN REL ATION TO NEOADJUVANT SYSTEMIC THERAPY
The use of US-guided FNA detection of disease in LNs prior to neoadjuvant chemotherapy and ALND at the time of surgery reveals a subset of patients who experience conversion of their axillary pathology from disease positive to disease negative following neoadjuvant therapy. This phenomenon is similar to that observed in the primary breast tumor with neoadjuvant chemotherapy. The reported rates of conversion in the axilla range between 23% and 25%, which raises questions about the ideal timing of initial axillary staging in relation to systemic therapy (18,19).
The timing of SLNB in those patients undergoing neoadjuvant chemotherapy varies between institutions and surgeons. Results from clinical trials such as the National Surgical Adjuvant Breast and Bowel Project B-18 demonstrate that those patients with no residual disease (pathologic complete response) in their breast and axilla have an improved prognosis (20,21). Thus, some surgeons and medical oncologists prefer to have SLNB information after neoadjuvant chemotherapy for prognosis, whereas other surgeons prefer performing SLNB prior to neoadjuvant chemotherapy because it provides accurate axillary staging information at initial presentation before the possible pathologic downstaging that occurs with therapy (22).
Performing SLNB after the completion of neoadjuvant chemotherapy would theoretically avoid ALND in those patients who convert their pathology from node positive to node negative following therapy. Multiple small series have examined SLNB after neoadjuvant chemotherapy and reported false-negative rates up to 33% (23), considerably higher than that observed when the procedure is performed prior to chemotherapy. This likely results from tumor necrosis and lymphatic fibrosis, consistent with studies demonstrating that patients after neoadjuvant chemotherapy tend to have fewer number of LN retrieved compared with the surgery-first patients (24,25), and assumes that all axillary node metastases will respond identically to chemotherapy. Issues relating to accuracy of SLNB after chemotherapy will be addressed in the ACOSOG Z1071 trial which is currently ongoing. The primary aim of this study is to determine the false-negative rate of SLNB after neoadjuvant chemotherapy. In this trial, patients with clinical stage II–III breast cancer and documented node-positive disease (as detected by FNA of radiographically abnormal appearing LNs or palpable lymph nodes) will undergo SLNB and cALND after completing chemotherapy.
The second timing dilemma related to SLNB is the timing of completion axillary dissection in patients with a positive SLNB and the issue is whether cALND should be performed concurrently with SLNB or at a second procedure. The ACOSOG Z0010 and Z0011 trials examine the effects of delayed versus immediate cALND in terms of surgical complications. These trials demonstrate that LN recovery and long-term complications were similar in both groups; however, short-term surgical complications were more common in the immediate cALND patients. cALND can be performed immediately if intraoperative analysis demonstrates metastatic disease in the SLN, but apart from the need for a second surgical procedure, there is no disadvantage in delaying the ALND until the final pathology is verified (26).
 INTRAOPERATIVE SLN EXAMINATION
INTRAOPERATIVE SLN EXAMINATION
The determination of SLN status intraoperatively has the advantage of performing definitive axillary surgery at the initial surgical procedure if the SLN contains metastatic disease. The most common methods to examine SLN intraoperatively are frozen section, imprint cytology, and molecular analysis. The use and accuracy of each of these techniques is dependent on the institution and the experience of the pathologists. The main disadvantage of frozen section is the complete loss of tissue and variable quality of the slides. Several studies have compared frozen with permanent sections for accuracy in detecting metastatic disease. The sensitivity of frozen section is reported to vary between 40% and 76% depending on the size of the metastatic deposit and the size of the primary tumor, making this procedure more effective for macrometastatic disease (27). Thus, routine frozen section may not be indicated in patients with small invasive cancers. Imprint cytology has the advantage of rapid processing, because no freezing or processing of tissue is required and because it does not involve the loss of pathologic tissue and it does not interfere with postoperative permanent section processing and analysis. Imprint cytology has been reported to have a 61% sensitivity and 100% specificity (28). Molecular analysis of SLN measures the mRNA expression levels of breast epithelial associated genes most commonly using polymerase chain reaction. There are two commercial devices in development which may become available for clinical use. The one-step nucleic acid amplification assay is an automated system for rapid detection of cytokeratin 19 mRNA. This procedure has been validated in a multicenter trial conducted in Japan. The Breast Lymph Node Assay detects the levels of cytokeratin 19 and mammoglobin mRNA using reverse transcriptase polymerase chain reaction. Both techniques have sensitivities >95% for LN metastases >2 mm and are comparable with permanent section pathology which makes them useful for intraoperative use (29–31).
 MICROMETASTATIC LN INVOLVEMENT
MICROMETASTATIC LN INVOLVEMENT
The American Society of Clinical Oncology guidelines for SLNB in early-stage breast cancer in 2005 stated that “SLNB is an appropriate initial alternative to routine staging ALND for patients with early-stage breast cancer with clinically negative axillary nodes. Completion ALND is still the standard treatment for patients with axillary metastasis identified on SLNB. Appropriately identified patients with negative results with SLNB when done under the direction of experienced surgeon, need not have completion ALND. Isolated cancer cells detected by pathologic examination of the SLN with the use of specialized techniques are currently of unknown clinical significance. Although such specialized techniques are often used, they are not a required part of SLN evaluation for breast cancer at this time” (32). Interest in micrometastatic disease and isolated tumor cell clusters (ITCs) detected in the SLN has grown since that report because of the increased detection rate from improvement in laboratory techniques.
Routine LN evaluation is performed using hematoxylin and eosin (H&E) staining. More sophisticated methods like immunohistochemical stains (IHC) for cytokeratins allow the identification of very small tumor deposits not readily apparent on H&E staining. According to the AJCC 2010 Cancer Staging Manual (seventh edition), micrometastasis is defined as tumor deposits greater than 0.2 mm but not greater than 2.0 mm in the largest dimensions (pN1mi). ITCs are defined as small clusters of cells not greater than 0.2 mm in largest dimension, and these cells are detected either by H&E or IHC staining. Patients with ITC only in their LNs are staged as pN0 (i+). The finding of tumor cells based on reverse transcriptase polymerase chain reaction technique stages patients as pN0 (mol+). These patients have positive molecular findings, but no regional LN metastases detected histologically.
Recent analysis of the US Surveillance, Epidemiology, and End Result database of 175,000 patients between 1990 and 2002 identified a 17.3% annual increase in the incidence of identification of micrometastatic LN involvement for patients with stage IIA and IIB breast cancer since 1997. The increase was noted more in estrogen receptor (ER)-positive compared with ER-negative disease (33). The increased detection of breast cancer in early stages results from improved screening mammographic techniques as well as the increased use of SLNB surgery and pathologic processing in the community practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree