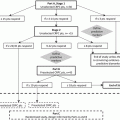
Castrate-resistant a prostate carcinoma with at least 1 of the following:
C1. Histologic evidence of small-cell prostate carcinoma (pure or mixed)
C2. Exclusively visceral metastases
C3. Radiographically predominant lytic bone metastases by plain x-ray or CT scan
C4. Bulky (≥5 cm) lymphadenopathy or bulky (≥5 cm) high-grade (Gleason ≥ 8) tumor mass in prostate/pelvis
C5. Low PSA (≤10 ng/mL) at initial presentation (before ADT or at symptomatic progression in the castrate setting) plus high volume (≥20) bone metastases
C6. Presence of neuroendocrine markers on histology (positive staining of chromogranin A or synaptophysin) or in serum (abnormal high serum levels for chromogranin A or GRP) at initial diagnosis or at progression. Plus any of the following in the absence of other causes: A. elevated serum LDH (≥2 × IULN); B. malignant hypercalcemia; C. elevated serum CEA (≥2 × IULN)
C7. Short interval (≤6 months) to androgen-independent progression following the initiation of hormonal therapy with or without the presence of neuroendocrine markers
Neuroendocrine (NE) Cells of the Normal Prostate
The epithelial compartment of the normal prostate gland consists of three types of epithelial cells—basal (proliferating), secretory (luminal), and NE cells interspersed within the basal cells. Neuroendocrine cells constitute <1 % of all the epithelial cells and are terminally differentiated. Therefore, NE cells in normal prostate typically lack proliferative activity and do not express the proliferation associated Ki-67 (MIB-1) antigen. Neuroendocrine cells are also AR negative, and thus do not express downstream AR target proteins including PSA [7–9]. Cellular extensions of NE cells in the form of neurite-like projections can help establish communication between NE cells and surrounding epithelial cells [10, 11]. This allows for effective paracrine signaling including secretion of products from a wide range of neuro-secretory granules present in NE cells, including serotonin, histamine, CgA, calcitonin gene family of peptides (calcitonin, katacalcin, and calcitonin gene-related peptide), neuropeptide Y, vasoactive intestinal peptide, bombesin, gastrin-releasing peptide (GRP), parathyroid hormone-related protein (PTHrP), NSE, thyroid-stimulating hormone-like peptide, vascular endothelial growth factor, and others [11]. This paracrine signaling is important in regulating the growth, survival, and differentiation of the surrounding normal prostate epithelial cells in an androgen-independent manner. Neuroendocrine cells can be isolated in normal prostate by specific immunostaining with antibodies against NSE, CgA, and synaptophysin [12, 13] and may be more prevalent in African Americans [14].
NED in Prostate Cancer
In clinically localized prostate adenocarcinoma, NE cells are typically focally distributed as single cells or in small nests amongst a predominant population of malignant prostate epithelial cells. On average, NE cells tend to constitute no more than 1 % of all tumor cells, except for the rare cases of small-cell prostate carcinoma or prostate carcinoid. The World Health Organization (WHO) has categorized NED in prostate cancer into three forms: (1) focal NED in conventional prostate adenocarcinoma; (2) carcinoid tumor (well-differentiated NE tumor); and (3) small cell NE carcinoma (poorly differentiated NE carcinoma) [15]. Depending upon the type of the detection methods utilized, the prevalence of NED in prostate cancers varies from 10 to 100 % and may carry prognostic significance [13, 16–20].
The amount of NED seen in prostate cancer increases with disease progression and with castration resistance (Fig. 21.1). Similarly, increased expression of CgA in the prostate and serum of patients on anti-ADT is commonly seen with disease progression [17]. Biopsies during stages of prostate cancer progression may often detect poorly differentiated tumors or those with mixed features containing both epithelial and neuroendocrine features. In a small subset of patients, transformation to predominantly androgen-independent NEPC can occur. At this transition, AR expression may be low or absent. This is often associated with clinical features of anaplastic prostate cancer (Table 21.1). The development of the clinically defined anaplastic phenotype predicts poor prognosis and response to platinum-based chemotherapy [21]. These tumors often are poorly differentiated, lack AR expression, and may show pure small cell or predominantly NE features.
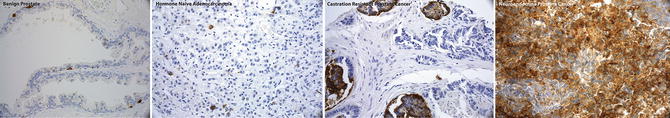

Fig. 21.1
Immunohistochemistry for Chromogranin showing representative examples of the spectrum of prostate cancer progression: Chromogranin IHC is negative in benign prostate (first panel), focally positive in hormone naïve prostate cancer (second panel), tends to increase in CRPC (third panel), and shows diffuse positive staining in NEPC (fourth panel)
Histology
In the setting of CRPC, NEPC typically refers to the presence of small cell carcinoma histology on tumor biopsy. Small cell carcinoma of the prostate consists of sheets and nests of small, rather uniform cells, with nuclear molding and scant cytoplasm. Nuclei are rounded and hyperchromatic, containing fine chromatin pattern and inconspicuous nucleoli. Mitoses are numerous, occurring at a rate of 5–10 per high-power field. Tumor necrosis is also common. The tumor tends to infiltrate widely and diffusely without well-defined margins. Lymphatic and blood vessel invasion are frequent. Large cell neuroendocrine carcinoma can also be seen but this is an extremely rare variant of NEPC. Large cell NEPC has an architecture that suggests NED. The tumor is composed of sheets and ribbons of large and polygonal cells with abundant, often eosinophilic, cytoplasm and nuclei with coarse chromatin and frequent nucleoli. The mitotic rate is high and necrosis is usually prominent. Rosette-like structures can be observed [22]. In approximately 50 % of cases, NEPC tumors are mixed with conventional adenocarcinoma [23, 24]. Today, the diagnosis of NEPC is primarily based on morphology but immunohistochemistry may be helpful in supporting the diagnosis. Neuroendocrine cancer cells are typically positive for one or more neuroendocrine markers such as synaptophysin, NSE, CgA, CD56, and are generally negative for AR and androgen-dependent markers such as PSA, prostatic acid phosphatase (PAP), and ETS-related gene (ERG). NEPC is negative for all neuroendocrine markers in approximately 10 % of cases. The diagnosis of metastatic NEPC may be challenging. In the case of a poorly differentiated metastatic adenocarcinoma, history of primary prostate cancer treated by long-term ADT, with early visceral metastasis and/or a normal or slightly elevated serum PSA level, clinicians should consider immunohistochemistry staining to evaluate for NEPC. In a patient that presents with small cell carcinoma of unknown primary, distinguishing prostatic small cell carcinoma from small cell carcinoma of another origin can also be challenging as they can appear morphologically similar and can both express common NE markers. Testing for the presence of the prostate-specific ERG gene fusion by FISH, which occurs in approximately 50 % of NEPC, can help confirm prostate origin and excludes neuroendocrine carcinoma from other primary sites [25, 26].
Molecular Mechanism of NEPC
The mechanisms and pathways that lead to NED in prostate cancer and the triggers of transformation to an AR low or AR negative, poorly differentiated or neuroendocrine phenotype in a patient with known adenocarcinoma of the prostate are poorly understood. It is believed that a synergistic functional network of pathways, rather than a single pathway is involved in the process of induction and sustenance of NED [27]. Androgen withdrawal is the most potent stimulator of NED in LNCaP prostate cancer cells, however various cytokines have also been reported to induce NED [28–32]. Neuroendocrine cells may also establish paracrine networks within the tumor and stimulate the proliferation of adenocarcinoma, invasion, metastasis, and progression to a castration-resistant stage. This likely is mediated through increased expression of receptors on the bulk non-NE/adenocarcinoma tumor cells for neuropeptides, biogenic amines, and cytokines secreted by the NE cells [10, 11, 33].
The addition of the cytokines interleukin-8 (IL-8) or interleukin-6 (IL-6) can induce NED in LNCaP cells by activating downstream signaling pathways involved in signal transduction, activation of transcription, mitogen-activated protein kinases, cyclic adenosine monopho-sphate-dependent protein kinase [34]. IL-6 can also activate phosphatidylinositol-3-kinase (PI3K)-dependent signaling pathways [35–37]. Activation of ERK and PI3K-AKT-mTOR signaling pathways along with androgen deprivation has also been reported to induce NED in LNCaP cells [38]. Cortes et al. reported that when LNCaP cells were treated with epidermal growth factor (EGF) in the presence of LY294002, an inhibitor of the PI3K-AKT pathway, an increase in the levels and activity of ErbB2 is observed. This finding was associated with cell survival and NE differentiation.
Overexpression of CXCR1, a receptor for IL-8, has been implicated in CRPC progression. CXCR2, which is homologous to CXCR1, is exclusively expressed on NE tumor cells. Normally the IL-8/CXCR2/P53 signaling pathway keeps the NE tumor cells in a quiescent state in an autocrine manner [39], and TP53 is critical in mediating this pathway. Loss of TP53 and/or other mechanisms resulting in dysregulation of the IL-8/CXCR2/P53 signaling pathway may represent a mechanism for NE cell proliferation [40]. Knockdown of TP53 using siRNA abolishes the growth inhibition of both LNCaP/CXCR2 and PC-3 cells by IL-8 [40, 41]. The role of TP53 in NEPC is further supported by the transgenic adenocarcinoma of mouse prostate (TRAMP) model, in which small cell prostate cancer develops as a result of SV40 T antigen inactivation of both TP53 and Rb. Similarly, Nikitin et al. developed a TP53−/−Rb−/− double knockout mouse model in which probasin promoter was used to drive the expression of the Cre-recombinase, and the resultant tumors exhibit a morphology similar to NEPC [42].
Recently, other genes and pathways have been implicated in the development of NEPC. Amplification of the cell cycle kinase, Aurora Kinase A (AURKA), and the transcription factor and oncogene, N-myc (MYCN) has been detected in NEPC tissues and thought to functionally cooperate to induce NEPC [43]. Importantly, this is potentially targetable using a small molecule aurora kinase inhibitor [43]. Loss of the REST transcriptional complex is another potential key driver in the development of NEPC [44]. Midkine (MDK) is a retinoic acid-induced heparin binding growth factor, highly expressed during embryogenesis is involved in neurogenesis and epithelial-to-mesenchymal transition (EMT). Its expression has been correlated with poor clinical outcomes in various human cancers, including prostate cancer [45–49]. Immunohistochemical analysis of MDK, the neuronal marker tubulin-beta III (TUBB3), and the NE-marker CgA was performed in 53 patients with PCa (hormone naïve and CRPC). Up-regulation of MDK, TUBB3, and CgA was observed in CRPC compared to hormone-naïve tumors. MDK expression was highly associated with the expression of both CgA and TUBB3, with MDK positive NE-like looking cells co-expressing CgA or, more commonly, CgA together with TUBB3. It was proposed that MDK up-regulation in CRPC is associated with NED (shown by its relation to CgA and TUBB3) and hypothesized as a potential target for prostate cancer therapy [50].
Clinical Presentation
Patients rarely present with de novo NEPC (<1 %), most commonly in form of small cell prostate cancer, and typically associated with metastatic disease at initial presentation. Unlike prostate adenocarcinoma, NEPC can often present with visceral metastasis (liver, brain) and/or lytic bone lesions. It can be challenging to distinguish NEPC from small cell carcinoma of other primary sites as they have similar histological and immunohistochemical features, i.e., absence of androgen-regulated genes and presence of neuroendocrine markers. In this case, determination of TMPRSS2-ERG gene rearrangement by FISH may prove helpful as it is present in about 50 % of prostate cancers but is universally absent in small cell cancers from other primary sites [25, 26]. Uncommonly, NEPC may also present with constitutional symptoms, hydronephrosis, bone pain, abdominal pain, hematochezia, or hematuria. Ectopic production of hormones such as adrenocorticotrophic hormone, antidiuretic hormone (ADH), thyroxine, etc., by the tumor may occasionally present as a paraneoplastic syndrome, with clinical manifestations of thyrotoxicosis, inappropriate ADH production, hypercalcemia, and/or adrenal hyperfunction. About 10 % of the patients with small cell NEPC present with paraneoplastic syndromes. Rarely, NEPC is included in multiple endocrine neoplasia 2A (Sipple syndrome), including malignant pheochromocytomas, thyroid medullary carcinomas, and parathyroid hyperplasia [51].
The role of NED markers in primary prostate adenocarcinoma as prognostic indicators is controversial. Some studies have found an association between NED and a worse prognosis [52–54], whereas others failed to find this relationship [55, 56]. There is a correlation between the immunohistochemically detected CgA and the serum levels of CgA [55, 57]. Serum CgA and NSE levels appear to be a useful indicator for detection of NED in patients with CRPC [58]. And elevated levels at earlier stages of the disease may indicate impending resistance to hormone therapy [59]. Following levels of serum markers such as CgA, NSE while on therapy has not shown correlation with response to therapy [57]. Carcinoembryonic antigen (CEA), a non-prostate-specific tumor marker [60, 61], is also often elevated in a subset of NEPC [62]. However, no obvious correlation has been detected between serum CEA levels and the levels of PSA and other NE markers.
Treatment
Despite being sensitive to both chemotherapy and radiotherapy similar to small cell lung cancer, most patients with NEPC or anaplastic prostate cancer only have a short-term response and eventually die within 1 year of diagnosis due to disease progression [63, 64]. Introduction of chemotherapy at early stages has shown some palliative benefit and a modest survival advantage. In one older study, a survival of 2 years in 20 % of patients was reported in patients receiving chemotherapy [65]. Rarely, transient remissions have been reported in patients who have received multimodality therapy with chemotherapy, surgery, and radiation.
As very few patients with NEPC present with localized disease at initial presentation, there is limited data on management of patients who present with organ confined disease. These patients are very likely to have occult metastatic disease not detected on bone scan or computerized tomography. Positron emission tomography (PET) may be useful in confirming localized disease. It is recommended to use a multimodality approach in treating these patients similar to small cell lung cancer, consisting of chemotherapy with concurrent or consolidative radiotherapy to control the local disease as well as systemic micro-metastasis [66]. Very limited data is available in context of use of surgical approach in this clinical setting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree