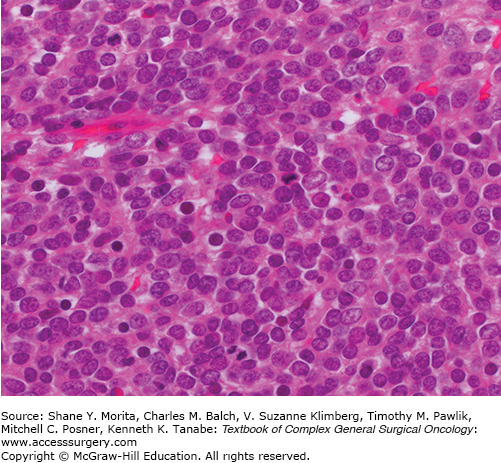
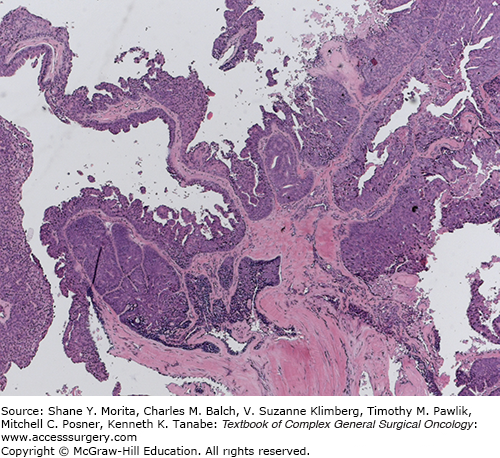
Merkel cell carcinoma (MCC) is an aggressive epidermal malignancy of neuroendocrine origin.1 While uncommon, affecting only 0.6 per 100,000 in the United States, its incidence appears to be increasing.2,3
MCC usually presents as a bland nonpigmented nodule on the head, neck, or extremities of older, fair skinned patients. The main risk factors are ultraviolet light exposure, age, and immune suppression.2,4 Merkel cell polyoma virus is integrated into the tumor genome in 80% of cases.5 The mechanism of viral oncogenesis remains the subject of investigation but the virus encodes large and small tumor antigens that are required for viral replication and tumorigenesis.6 Histologically, MCC is composed of uniform small round cells with basophilic nuclei and minimal cytoplasm (Fig. 21-1).
MCC is characterized by high rates of local recurrence as well as regional and distant metastatic spread. The first consensus staging system was introduced by the American Joint Committee on Cancer (AJCC) in 2010 and classifies MCC as stage I/II if it is localized to the primary site (stage I ≤2-cm size, stage II >2-cm size), stage III if there are regional node metastases, and stage IV if there is spread to distant sites.7,8 Overall prognosis is poor with 5-year disease-specific survival for patients with stage I/II disease 64%, stage III 39%, and stage IV 18%.8 Primary tumor thickness also correlated with survival in a recent analysis of 95 patients9 though results of earlier studies provide conflicting evidence. Fluorodeoxyglucose positron emmission tomography/computed tomography (FDG-PET/CT) is increasingly utilized in the staging of MCC. A retrospective series demonstrated that FDG-PET altered the staging of patients with MCC in 22% of cases and resulted in a management change in 37%.10
The management of MCC requires a multidisciplinary approach that incorporates surgery, radiotherapy, and chemotherapy, and takes into consideration disease stage, tumor site, and patient fitness.
Management of primary MCC has traditionally been surgical with wide excision to fascia with 1- to 3-cm margins, aiming to obtain histologically clear margins when clinically feasible.11,12 However, the management of MCC is increasingly controversial because the tumor is highly radiosensitive1 and there is a growing evidence base for the use of radiotherapy as both adjuvant and definitive treatment.
In the adjuvant setting, a review of the literature demonstrated that local recurrence rates were significantly lower after adjuvant primary site radiotherapy (10.5%, range 0% to 33%) than surgery alone (52.6%, range 6% to 100%).13 This is supported by a study from Sydney in which MCC was 0.39 times less likely to recur locally after adjuvant radiotherapy14 and a US SEER database analysis,15 but a series from the Memorial Sloane Kettering Cancer Center demonstrated excellent local control rates from surgery alone and no additional benefit from adjuvant radiotherapy.16
Primary tumor site management using definitive radiotherapy alone has also been reported, with excellent local control for patients with macroscopic disease or margin involvement after excision biopsy. A series of 26 patients reported 89% in-field control at 2 years17 and this approach may be particularly useful when anatomic and aesthetic considerations make wide excision challenging.
The role of sentinel node biopsy (SNB) in assessing the regional node field and the management of the regional node field in the management of MCC are also contentious issues.18 Approximately 30% of patients with clinically localized MCC will have a positive SNB19 and SNB is likely to provide valuable prognostic information, with worse outcomes reported in SNB-positive patients.16 However, the biologic behavior of MCC differs from that of melanoma and breast cancer, where SNB is now considered standard management. High failure rates (5% to 33%)20 and false negative rates (20%)21 have been reported after SNB for MCC and it is possible that the most important role for lymphoscintigraphy in MCC is in planning radiotherapy fields, rather than in enabling SNB.
In patients with high-risk MCC (where high risk was defined as a primary tumor greater than 1-cm diameter, gross residual disease after surgery, local recurrence, or involved regional nodes), treatment with combined chemoradiation resulted in overall survival, local control, and distant control rates of 76%, 75%, and 76% at 3 years, respectively.22
The first and only randomized control trial involving MCC patients assessed the role of adjuvant prophylactic regional radiotherapy in patients with clinically localized MCC.23 The study closed early due to poor accrual but 83 patients all underwent primary site wide excision and radiotherapy and were randomized to receive regional radiotherapy or not. There was a significant reduction in regional recurrence in the patients who received regional radiotherapy (16.7% vs. 0%, p = 0.007) but no difference in overall survival. This trial strongly suggests the benefit of regional radiotherapy in clinically node-negative MCC patients in terms of regional control and, though this is currently not recommended in clinical guidelines,11,12 regional radiotherapy should be considered in MCC patients even after a negative SNB.
Regional node-positive (stage III) MCC is treated with radical lymphadenectomy and/or radiotherapy.11,12 There is some evidence that adjuvant radiotherapy after lymphadenectomy reduces the risk of regional recurrence and some studies hint at a survival benefit but all are too small to provide definitive evidence.13 Troublesome in-transit MCC metastases of the limb may be managed using isolated limb perfusion or isolated limb infusion with cytotoxic drugs.24,25
Cutaneous adnexal tumors (CATs) are rare neoplasms of the skin that are derived from the adnexal structures: hair follicles, sebaceous glands, apocrine glands, and eccrine glands. They vary in character from benign to malignant. The majority of cases are benign (94.3% in one large series) and the most common benign CAT histotypes are: eccrine spiradenoma, hidrocystoma, eccrine poroma, syringoma, sebaceous adenoma, and trichofolliculoma. Malignant CATs include sebaceous carcinoma, eccrine carcinoma (Fig. 21-2), and apocrine carcinoma.27,28
The diagnosis of a benign or malignant sebaceous tumor should prompt investigation for the Muir-Torre syndrome. This autosomal dominant cancer predisposition syndrome is characterized by sebaceous tumors (adenomas or carcinomas) and visceral tumors (usually colorectal or endometrial). In a subset of patients, Muir-Torre syndrome is due to germline mutations in the mismatch repair genes hMSH2 and hMLH1. Immunohistochemical staining of the sebaceous tumor for loss of expression of mismatch repair proteins can be utilized to identify patients who should be considered for germline mutation testing. In patients with germline mutations, Muir-Torre syndrome is considered a subtype of Lynch Syndrome and cancer predisposition arises due to microsatellite instability. Identification of Muir-Torre syndrome patients allows appropriate screening and surveillance.29
Benign CATs are treated by complete surgical excision. There is no consensus treatment algorithm for malignant CATs. Most centers recommend wide excision of the primary site down to fascia. A retrospective review of 48 malignant CAT cases documented local recurrence in 18.8% of cases and regional node recurrence in 8.3%. All the patients who developed regional recurrence had first developed local recurrence. The authors concluded that any role for SNB may be limited to those patients who develop local recurrence. The 5-year disease-specific survival was 97%.30
Dermatofibrosarcoma protuberans (DFSP) is a low-grade malignant dermal tumor of fibroblast origin that can affect both adults and children and has an incidence of 0.4 per 100,000 in the United States.31 It accounts for 18.4% of cutaneous sarcomas.32
DFSP most commonly presents as a slow-growing plaque on the head and neck or an extremity. Histology demonstrates bland CD34-positive spindle cells with little cytological atypia in a storiform arrangement, which typically infiltrate the subcutis in a “honey comb” pattern (Fig. 21-3). However, fibrosarcomatous (-FS) transformation can occur resulting in greater cellularity, higher mitotic activity, cytological atypia, a herring bone architecture, and loss of CD34 expression (DFSP-FS). In most cases, DFSP is characterized by a specific chromosomal translocation t(17:22), or a supernumerary ring chromosome, that results in overexpression of platelet-derived growth factor ß.33
FIGURE 21-3
Dermatofibrosarcoma protruberans.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree