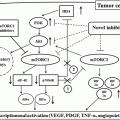
Treatment
Number of patients
Line of treatment
ORR (%)
PFS (months)
OS (months)
903
Second line
10 vs. 2
5.5 vs. 2.8 (p < 0.01)
17.8 vs. 15.2 (p = 0.146)
Temsirolimus vs. sorafenib [15]
512
Second line
20 vs. 20
4.3 vs. 3.9 (p = 0.19)
12.3 vs. 16.6 (p = 0.01)
750
First line
47 vs. 12
11 vs. 5 (p = 0.0001)
26.4 vs. 21.8 (p = 0.051)
Axitinib vs. sorafenib [45]
723
Second line
23 vs. 12 (p = 0.0001)
8.3 vs. 5.7 (p < 0.0001)
20.1 vs 19.2 (p = 0.3744)
Axitinib vs. sorafenib [46]
288
First line
32 vs 15 (p = 0.0006)
10.1 vs. 6.5
Not reported
435
First or second line
30 vs. 3 (p < 0.001)
9.2 vs. 4.2 (p < 0.0001)
22.9 vs. 20.5 (p = 0.224)
Pazopanib vs. sunitinib [53]
1110
First line
31 vs 25 (p = 0.03)
8.4 vs. 9.5 (HR = 1.05; 95 % CI, 0.90–1.22)
28.4 vs. 29.3 (HR = 0.91, 95 % CI, 0.76–1.08)
10.2 Sorafenib
Sorafenib (Nexavar™, Bayer Pharmaceuticals, West Heaven, CT, and Onyx Pharmaceuticals, Emeryville, CA) is an oral anti-VEGFR agent and the first one to be approved by the FDA in December 2005 for the treatment of metastatic renal cell carcinoma. It is a bis-aryl urea with broad anti-tyrosine kinase activity, including VEGFR-2, VEGFR-3, PDGFR-β, RAF-1, wild-type and V599E-mutated BRAF, FLT-3, c-KIT, and FGFR-1 [7].
10.2.1 Clinical Efficacy
10.2.1.1 Phase I
Four phase I studies have assessed the safety and tolerability of different dosing and treatment schedules of sorafenib in solid tumors [8–11]. The initial study [8] included 69 patients with advanced and refractory tumors that were treated with escalating doses of sorafenib, ranging from 50 to 800 mg orally once or twice daily continuously. The most common treatment-related adverse events were diarrhea, fatigue, skin toxicities, anorexia, and nausea, and most of them resolved upon treatment withdrawal. Significant dose-limiting grade 3 toxicities were diarrhea, fatigue, and hand-foot syndrome. The maximum tolerated dose from this trial was 400 mg twice daily. The only renal cell carcinoma patient that was included achieved stable disease for more than 2 years. Different treatment schedules in other phase I studies include 7 days on treatment followed by 7 days off treatment [9], 21 days on treatment followed by 7 days off treatment [10], and 28 days on treatment followed by 7 days off treatment [11]. Since the continuous treatment schedule was associated with good tolerance and stable blood concentrations of sorafenib, the recommended dose and schedule for subsequent phase II and III studies were 400 mg twice daily.
10.2.1.2 Phase II
A phase II randomized discontinuation trial evaluated the activity and tolerability of sorafenib in 202 patients with metastatic RCC [12]. Patients were allowed to enroll regardless of clear cell histology and previous treatment. All patients received sorafenib 400 mg twice daily for a total of 12 weeks. Following that initial treatment period, patients were assessed for response. Subsequently, they were either randomized to treatment with sorafenib or placebo if the change in the bidimentional size of the tumor was ≤25 %, or continued sorafenib if the tumor had shrunken by ≥25 %, or discontinued treatment if there was tumor growth of ≥25 %. The primary endpoint of the trial was percentage of the randomized patients that remained free from disease progression at 24 weeks of treatment, while secondary endpoints included progression free survival (PFS) for the randomized group, PFS for the entire cohort, objective response rate (ORR), and safety. After the initial 12 weeks of treatment, 69 patients (34 %) met the criteria for randomization, whereas 73 patients (36 %) had tumor shrinkage of ≥25 % and continued on sorafenib, and 51 patients (25 %) discontinued treatment due to either tumor growth of ≥25 % or other evidence of progression at or before week 12 of treatment. From the 69 eligible patients, 65 patients were randomly assigned to sorafenib (n = 32) or placebo (n = 33). Between the two arms, more patients that received sorafenib were disease free at the end of the 24 weeks compared to placebo (16/32, 50 % vs. 6/33, 18 %; p = 0.0077). Also the median PFS between the two groups was in favor of sorafenib (24 vs. 6 weeks; p=0.0087). The median PFS for the entire cohort was 29 weeks. The most common treatment-related adverse events were fatigue, skin toxicities, pain, and diarrhea, with most of them being grade 1 or 2. The most common grade 3 or 4 adverse event was hypertension.
10.2.1.3 Phase III
The pivotal phase III TARGET trial examined the role of sorafenib as second-line treatment in cytokine-refractory patients [13]. Nine hundred three patients with metastatic clear cell RCC that had previously failed treatment with cytokines were randomly assigned 1:1 to receive either sorafenib 400 mg twice daily or placebo. Most patients had prior nephrectomy, and all of them had either good or intermediate-risk disease by MSKCC criteria. The primary endpoint of the study was overall survival (OS), whereas secondary endpoints included PFS and ORR. In the initial interim analysis and prior to crossover to sorafenib, the median OS was significantly prolonged for sorafenib compared to placebo (not reached vs. 14.7 months, respectively; p = 0.02). As a result of those favorable results, crossover to sorafenib was allowed for the patients that progressed on placebo. In the final analysis [14], there was a trend toward improved median OS for the sorafenib arm (17.8 vs. 15.2 months; p = 0.146); however, when post-crossover placebo survival data were censored, the difference was found to be statistically significant (17.8 vs. 14.3 months; p = 0.029). The median PFS was also found to be in favor of sorafenib (5.5 vs. 2.8 months; p < 0.000001). Common side effects were grade 1 or 2 and included diarrhea, fatigue, skin toxicities, nausea, and hypertension. The incidence of cardiac ischemia and myocardial infarction was higher in the sorafenib group compared to placebo (3 % vs. <1 %, respectively; p=0.01), including two deaths in the sorafenib group and one death in the placebo group. Finally, baseline VEGF levels were found to be a negative prognostic biomarker for PFS and OS (p = 0.0013 and p = 0.0009, respectively); however, they were not accurate predictive markers for response, since patients with either high or low baseline levels benefited from therapy with sorafenib. This trial established the first proof of principle that targeting VEGFR could lead to improved clinical outcomes in metastatic RCC patients.
The recently published INTORSECT phase III trial compared temsirolimus to sorafenib in the second-line setting in patients previously treated with sunitinib [15]. A total of 512 patients were 1:1 randomized to either intravenous temsirolimus 25 mg weekly (n = 259) or oral sorafenib 400 mg twice daily (n = 253). Both patients with clear cell and non-clear cell histologies were enrolled. The primary endpoint of PFS was not significantly different between the two groups (median PFS of 4.3 months for temsirolimus vs. 3.9 months for sorafenib; p = 0.19); however, the secondary endpoint of OS between the two arms favored sorafenib (median OS of 12.3 months for temsirolimus vs. 16.6 months for sorafenib; p = 0.01). Exploratory subgroup analyses showed favorable OS with sorafenib in patients with prior nephrectomy (p = 0.002), clear cell histology (p = 0.01), prolonged exposure to sunitinib for more than 180 days (p = 0.02), and intermediate MSKCC risk (p = 0.002). The most common side effects with temsirolimus were skin rash, fatigue, cough, anemia, and nausea, whereas the most common side effects with sorafenib were diarrhea, HFS, decreased appetite, skin rash, and fatigue. The reason for the discrepancy between PFS and OS is unclear, but these results have reinforced the practice of sequential VEGFR inhibitors in the treatment sequence for metastatic RCC patients.
Finally, the recently presented SWITCH phase III trial aimed to compare the sequential use of sorafenib and sunitinib vs. sunitinib and sorafenib in previously untreated patients [16]. Once 182 patients were assigned to the sorafenib/sunitinib arm vs. 183 patients to the sunitinib/sorafenib arm. The primary endpoint of total PFS from the time of randomization to disease progression on the second VEGFR agent was not statistically different between the two groups (12.5 months in the sorafenib/sunitinib arm vs. 14.9 months in the sunitinib/sorafenib arm; p = 0.54). Median OS likewise showed similar results, reaching 31.5 months in the sorafenib/sunitinib arm vs. 30.2 months in the sunitinib/sorafenib arm (p = 0.49). Even though this trial enrolled patient with low or intermediate MSKCC score and captured the OS after sequential treatments, the median OS is among the longest yet reported, further supporting the use of sequential VEGFR inhibitors. Unfortunately the trial was underpowered to show significant differences and thus is unlikely to impact clinical practice.
10.3 Sunitinib
Sunitinib (Sutent™, Pfizer Inc., New York, NY) is an oral, small-molecule, multitarget tyrosine kinase inhibitor, including VEGFR-1, VEGFR-2, and VEGFR-3, PDGFR-α and PDGFR-β, c-KIT, FLT-3, CSF-1R, and RET [17]. It is the most widely used agent for metastatic RCC, and it has also been approved by the FDA for the treatment of imatinib mesylate-refractory gastrointestinal stromal tumor (GIST) and locally advanced or metastatic well-differentiated pancreatic neuroendocrine tumors (pNET).
10.3.1 Clinical Efficacy
10.3.1.1 Phase I
Several phase I studies of sunitinib have been conducted in patients with both solid tumors and acute myelogenous leukemia [18–20]. The phase I trial in solid tumors [18] included 28 patients that were treated with escalating oral doses of sunitinib, ranging from 50 mg every other day to 150 mg daily for 4 weeks in a 6-week cycle. Significant dose-limiting toxicities were grade 3 fatigue and hypertension and grade 2 bullous skin toxicity, all of them reported at doses ≥75 mg daily. Based on those results, the recommended dose for subsequent studies was 50 mg daily for 4 weeks followed by a 2-week break in a 6-week cycle (schedule 4/2). At this dose level, the most common side effects were sore mouth, edema, thrombocytopenia, hair discoloration, and yellow coloration of the skin. A total of four patients with metastatic RCC were included, with three of them showing objective responses lasting from 28 to 54 weeks.
10.3.1.2 Phase II
Two consecutive phase II clinical trials examined the role of sunitinib in the treatment of metastatic RCC refractory to cytokines [21, 22]. Sixty-three and 106 patients with metastatic RCC were enrolled in those two studies, respectively. In both trials, patients were required to have an ECOG performance status of 0 to 1, and more than half of them had 0 MSKCC risk factors (n = 34, 54 % and n = 61, 58 %, respectively). The first study allowed patients with non-clear cell histologies to enroll; however, the patients in the second trial were exclusively clear cell RCC. All patients were treated with sunitinib 50 mg daily for 4 weeks followed by a 2-week break in 6-week cycles. The primary endpoint of ORR was 40 % and 34 % between the two studies, whereas the secondary endpoints of median time to progression (TTP) and median OS were 8.7 and 16.4 months in the first study and 10.7 and 23.9 months in the second study, respectively [23]. The most common side effects encountered were fatigue, diarrhea, dyspepsia, nausea, and hypertension. Based on these results, sunitinib was granted accelerated approval by the FDA for the treatment of metastatic RCC in January 2006 [24].
Even though sunitinib was approved for treatment of RCC based on those two trials, questions regarding the optimal treatment schedule remained. The EFFECT trial assessed the efficacy and safety of two different schedules of sunitinib with the aim to identify the optimal dosing and schedule [25]. Two hundred ninety-two patients with treatment-naïve advanced clear cell RCC were 1:1 randomly assigned to sunitinib 50 mg daily 4/2 (n = 146) or 37.5 mg daily continuously (n = 146). Although this trial was small and underpowered to establish differences in outcomes, the 4/2 schedule correlated with a numerically longer TTP (9.9 months in the 4/2 schedule vs. 7.1 months in the continuous treatment arm; p = 0.09) and no higher rates of toxicity. Steady-state plasma trough concentrations of sunitinib, its active metabolite SU12662, and total drug were higher in the 4/2 arm; however, there was no correlation between drug plasma concentrations and objective tumor response with either dosing schedule.
Based on the above results the recommended dose of sunitinib remains 50 mg daily 4/2. However, in clinical practice, maintenance of sunitinib dose at 50 mg daily 4/2 can be challenging due to treatment-related adverse events, frequently requiring dose reductions and treatment breaks. Since worsening toxicities are usually observed during weeks 3 and 4 of treatment, several retrospective studies have examined the efficacy, safety, and tolerability of alternative treatment schedules [26–28]. Sunitinib 50 mg daily for 2 weeks followed by a 1-week break seems to be a reasonable alternative, with better tolerance and similar – if not improved – clinical efficacy. Ongoing prospective studies will provide more insight into the evolving field of optimal dosing schedule of sunitinib.
Sunitinib has also been compared to everolimus as a first-line treatment in patients with metastatic RCC. The phase II non-inferiority RECORD-3 trial [29] enrolled 471 patients with metastatic RCC (both clear and non-clear cell) that were randomized 1:1 to either everolimus followed by sunitinib upon disease progression (n = 238) or sunitinib followed by everolimus upon disease progression (n = 233). The primary endpoint of non-inferiority of everolimus was not reached, since the median PFS of first-line sunitinib was 10.7 months vs. 7.9 months with everolimus (HR 1.43, 95 % CI 1.15–1.77). Also, there was a trend toward an inferior OS in patients that received everolimus followed by sunitinib compared to sunitinib followed by everolimus (22.4 vs. 32 months; HR = 1.24, 95 % CI 0.94–1.64); however, these data are still immature and further follow-up is indicated. These data reinforce the clinical practice of frontline therapy with a VEGF inhibitor in metastatic RCC.
10.3.1.3 Phase III
A subsequent multicenter randomized phase III trial compared sunitinib to interferon-α (IFN-α), the standard of care at the time [30]. Seven hundred fifty treatment-naïve patients with metastatic clear cell RCC were assigned 1:1 to either oral sunitinib 50 mg daily for 4 weeks every 6 weeks (n = 375) or subcutaneous INF-α 9 million units three times weekly (n = 375). An ECOG performance status of 0 to 1 and absence of brain metastasis were required for study entry. Most patients had undergone prior nephrectomy (91 vs. 89 %). In addition, most patients had MSKCC favorable (38 vs. 34 %) or intermediate (56 vs. 59 %) risk disease with only a minority having poor risk disease (6 vs. 7 %). The primary endpoint of median PFS was 11 months for the sunitinib arm compared to 5 months for the IFN-α arm (p<0.001). In addition, sunitinib was associated with a higher ORR of 31 % vs. 6 % for IFN-α (p<0.001). All grades’ adverse events were more common in the sunitinib arm with the most common being diarrhea, fatigue, nausea, vomiting, and hypertension in comparison to fatigue, pyrexia, nausea, chills, and myalgias in the group treated with IFN-α. However, patient-reported health-related quality of life was significantly better in the sunitinib group (p < 0.001).
In a follow-up report of that trial [31], median OS of the patients that received sunitinib was greater compared to IFN-α (26.4 vs. 21.8 months; p=0.51). One possible explanation for the borderline p value is the high rates of crossover to sunitinib for the patients that progressed on IFN-α. When the analysis was censored to exclude those patient, the difference in the median OS was found to be statistically significant (26.4 vs. 20.0 months; p=0.36).
10.3.1.4 Expanded-Access Trial
Since several criteria were required for participation to the initial trials with sunitinib, a subsequent expanded-access trial was undertaken in order to assess the safety and efficacy of sunitinib in patients that did not meet the prespecified criteria [32]. Patients with an ECOG performance status of ≥2, brain metastasis, age ≥65 years, and non-clear cell histology were allowed to participate. More than 4500 patients were treated with sunitinib 50 mg daily on a 4/2 schedule in a compassionate basis, with 4371 of them included in the intention-to-treat (ITT) cohort. For that cohort, the ORR was 17 %, the median PFS was 10.9 months (95 % CI 10.3–11.2), and the median OS was 18.4 months (95 % CI 17.4–19.2). This trial confirmed the activity and safety of sunitinib in an unselected patient population.
10.4 Axitinib
Axitinib (Inlyta ™, Pfizer Inc., New York, NY) is an orally bioavailable second-generation indazole derivative. It is a potent small-molecule tyrosine kinase inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3 at picomolar concentrations and PDGFR-α and PDGFR-β and c-Kit inhibitor in nanomolar concentrations [33].`Thus, in therapeutic plasma concentrations, it blocks VEGF-mediated tumor vascularization by a number of signaling processes that promote the proliferation, migration, and survival of endothelial cells [34].
10.4.1 Clinical Efficacy
10.4.1.1 Phase I
The initial phase I study of axitinib [35] included 36 patients with advanced solid tumors refractory to standard therapy. Participants were treated with escalating doses of axitinib, ranging from 10 mg to 60 mg daily divided to 2 oral doses. Significant dose-limiting toxicities reported were hypertension, hemoptysis, and stomatitis with hypertension, fatigue, nausea, and diarrhea being the most common side effects observed. The recommended dose of axitinib from that trial was 5 mg twice daily in the fasted state. Six patients with metastatic RCC were included with two of them achieving a partial response (PR). Subsequent phase I studies in Japanese patients with advanced solid malignancies have shown similar pharmacokinetic and safety results and have also confirmed the effect of axitinib to soluble VEGFR-2 levels [36, 37].
10.4.1.2 Phase II
Using the recommended dose from the phase I studies, three subsequent phase II trials assessed the safety and efficacy of axitinib in patients with metastatic RCC who had failed previous therapies [38–41]. The initial single-arm, open-label phase II study allowed patients refractory to prior cytokine-based therapies to participate [38]. Patients were required to have an ECOG performance of 0 to1, measurable disease by RECIST, and no prior exposure to antiangiogenic agents. Fifty-two patients were treated with axitinib starting at 5 mg twice daily. The primary endpoint of the study was ORR, and the secondary endpoints were duration of response, TTP, OS, safety, pharmacokinetics, and patient-reported health-related quality of life. The median age of the cohort was 59 years, most patients (94 %) had prior nephrectomy, and all but one (98 %) had clear cell histology. The ORR was 44.2 % [2 complete responses (CRs) and 21 partial responses (PRs) (95 % CI, 30.5–58.7)] with a median response duration of 23.0 months (95 % CI, 20.9, not estimable; range 4.2–29.8). Twenty-two additional patients (42 %) showed stable disease (SD) for more than 8 weeks. Further, the median TTP was 15.7 months (95 % CI 8.4–23.4), and the median OS was 29.9 months (95 % CI 20.3,not estimable; range 2.4–35.8). The most commonly reported side effects were diarrhea, hypertension, fatigue, nausea, and hoarseness. A recent update of that trial [39] showed a 5-year survival rate of 20.6 % (95 % CI, 10.9 %–32.4 %). A pharmacokinetic post hoc analysis aiming to associate drug levels with efficacy was also undertaken. Patients were stratified to 4 quartiles based on their axitinib plasma concentration 1 to 2 hours post-dose on day 1 of cycle 1, with the third group exhibiting a numerically prolonged median PFS and OS. The biologic explanation was that a higher concentration of axitinib that is associated with good tolerance and no discontinuation of therapy is associated with optimal efficacy.
A subsequent phase II study evaluated the efficacy and safety of axitinib in sorafenib-refractory metastatic RCC patients [40]. Sixty-two eligible patients were enrolled in this multicenter, single-arm, open-label phase II study. All patients had disease progression while receiving sorafenib with most of them (74.2 %) having received more than one lines of treatment, including sunitinib and bevacizumab. The primary endpoint was ORR, and the secondary endpoints were safety, duration of response, PFS, OS, and patient-reported outcomes. The starting dose of axitinib was again 5 mg twice daily, and if well tolerated, a stepwise dose titration to 7 and 10 mg twice daily was undertaken. The median age of the cohort was 60 years, all patients had undergone prior nephrectomy, and the predominant histology was clear cell (95.2 %). The reported ORR was 22.6 % (95 % CI, 12.9–35.0 %) with a median duration of response of 17.5 months (95 % CI, 7.4, not estimable), a median PFS of 7.4 months (95 % CI, 6.7–11.0), and a median OS of 13.6 months (95 % CI, 8.4–18.8). Thirty-three patients (53.2 %) were able to tolerate increased doses of axitinib, whereas 11 patients (17.7 %) required dose reduction to less than 5 mg twice daily. The most common all-causality grade 3 to 4 side effects were hand-foot syndrome, fatigue, hypertension, dyspnea, diarrhea, dehydration, and hypotension.
Another single-arm, open-label, multicenter phase II evaluated the safety and efficacy of axitinib in Japanese patients who had failed prior cytokine therapy [41]. Dose titration to 7 and 10 mg twice daily was again recommended if no hypertension developed and axitinib was well tolerated. Sixty-four eligible patients were enrolled. The reported ORR and median PFS were 50.0 % (95 % CI, 37.2–62.8) and 11.0 months (95 % CI, 9.2–12.0), respectively, confirming the favorable response to axitinib. Commonly reported side effects were again hypertension, hand-foot syndrome, diarrhea, and proteinuria.
Based on the results from the phase I study, patients receiving axitinib exhibit variable plasma drug exposure with dose-proportional pharmacokinetics suggesting higher plasma exposure with escalated doses [35]. Further, higher axitinib exposure has been associated with prolonged PFS and OS [39, 42]. Based on that rationale, a randomized, double-blind, multicenter, phase II study that examined the efficacy of dose titration of axitinib was undertaken [43]. Two hundred thirteen previously untreated metastatic RCC patients received axitinib 5 mg twice daily as the initial treatment. One hundred twelve patients who did not experience elevation in their blood pressure (>150/90 mmHg) or any grade 3 or 4 side effects were subsequently randomized to either increased dose of axitinib to 7 mg twice daily with a further increase to 10 mg twice daily or placebo, while the rest of the patients continued treatment on standard dose. Even though the ORR was higher in the axitinib titration group compared to placebo (54 vs. 34 %, p = 0.01), no difference in the median PFS was observed between the two groups (14.5 vs. 15.7 months; p = 0.24). No significant differences in the all-causality serious adverse events between the two groups were reported. These result support that dose titration of axitinib can lead to clinical benefit (improved ORR), but the precise schema for doing so requires further study, as tolerance issues in the titration group likely prevented an adequate duration of exposure to the higher dose. Smaller increments in dose and alternative parameters for titration may improve outcomes further. This is an evolving field that further studies regarding the timing and scheme of titration are indicated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree