Salivary gland (SG) tumors are rare neoplasms of the neck and head which can occur in the paired major salivary glands or in one of the hundreds of minor salivary glands distributed throughout the oral cavity and pharynx. Benign neoplasms are slow growing, amenable to surgical cure, and if appropriately excised recur with a low frequency. Malignant tumors can arise from any one of the cellular components of the SG. They are generally faster growing compared to their benign counterparts and often invade into adjacent structures. A subset of malignant tumors display substantial predilection for nerve invasion which can lead to increased patient morbidity and a lower rate of surgical cure. For high-grade histologies clinical outcomes remain poor and multimodality treatment strategies are employed at most centers.1–5 Due to the rarity of each specific malignant histology, there is a dearth of prospective clinical trial data, which limits our understanding of disease progression and the effects of specific surgical and nonsurgical interventions. A more detailed molecular characterization of SG cancers may shed additional light on the pathogenesis of this disease process and provide novel therapeutic interventions in the future.
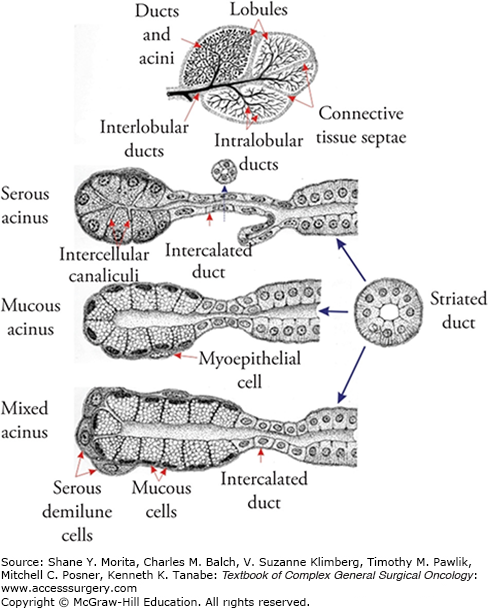
Salivary glands are exocrine glands arranged around acini composed of secretory cells.6 Serous, mucoserous, and mucous acini are organized around ductal structures which coalesce into larger secretory ducts (Fig. 58-1). The major SGs include two parotid glands, two submandibular glands (SMGs), and the sublingual glands. The parotid glands are primarily serous in nature and their secretions empty into the oral cavity via Stensen’s duct. One unique feature of the parotid gland is its intricate relationship with the facial nerve, which enters the gland on its posterior aspect and divides the gland into a superficial and a deep lobe. Although these terms are commonly utilized in the surgical literature, there are no fascial planes dividing the two lobes of the parotid gland (Fig. 58-2). The SMGs are found in close proximity to the lingual and hypoglossal nerves and secrete a combination of serous fluid and mucus which reaches the oral cavity through Wharton’s duct. Under baseline conditions, most salivary volume is produced by the SMGs, although under conditions of stimulation, the parotid glands can account for most surplus salivary capacity. The sublingual glands reside in close proximity to the opening of Wharton’s duct and empty primarily mucous secretions into the oral cavity through multiple excretory ducts. In addition to the major salivary glands, there are hundreds of minor SGs distributed throughout the oral cavity, including lip, soft and hard palate, floor of mouth, and buccal mucosa.6
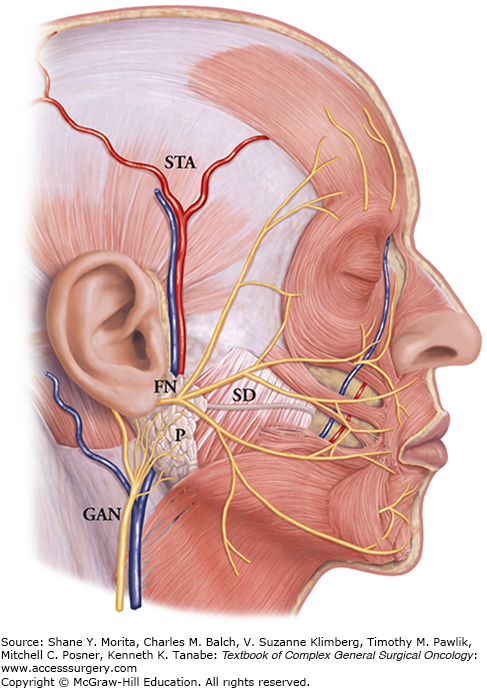
FIGURE 58-2
Parotid gland anatomy. Abbreviations: STA, superficial temporal artery; GAN, greater auricular nerve; SD, Stensen’s duct; P, parotid; FN, facial nerve). (Adapted with permission from Seckel B and Greene AB. Facial Danger Zones: Avoiding Nerve Injury in Facial Plastic Surgery. St. Louis, MO: Quality Medical Publishing Inc; 1994.)
Salivary gland function is essential to the homeostasis of the oral cavity. Perturbations in salivary production lead to altered bacterial load, impaired mucosal wound healing, and over time, poor dental health.7,8 These factors are often encountered during the treatment of head and neck malignancies, particularly when salivary glands are exposed to external beam radiotherapy (EBRT). Treatment-related xerostomia is one of the most common, and one of the more debilitating side effects of EBRT, particularly when combined with surgical resection in the context of salivary gland malignancy.
Benign salivary gland tumors are ten times more common than their malignant counterparts.6,9 The two most commonly encountered benign SG neoplasms are pleomorphic adenoma (PA) and Warthin’s tumor (WT) which together, account for approximately 70% of salivary gland tumors. PA occurs most commonly in the fourth decade of life, with a slight female preponderance. WT is the second most common benign salivary gland tumor in most countries although it may be less common in African Americans. It most commonly occurs in the sixth decade of life and is very rare in young people. Over the last half decade the male to female ratio has dropped from approximately 10:1 to almost 1:1.9 The precise driving force behind this change is unclear. However, in view of the established correlation between smoking and WT, it may reflect changes in the male to female ratio among smokers. Wang et al10 described the epidemiology of salivary gland tumors in the Chinese population and similarly found PA and WT to be the most common benign salivary gland tumors. Unlike PA which, are usually unilateral and isolated, WT is often bilateral and multifocal. Other benign histologies include basal cell adenoma, myoepithelioma, canalicular adenoma, sebaceous adenoma, and lymphadenoma.
Most benign tumors are commonly encountered in the parotid gland (80% for PA and about 90% for WT) and display a slow rate of growth, without invasion of adjacent structures but with pushing borders which can result in cosmetic deformity.6,9 When tumors are present in the deep lobe of the parotid they can cause functional impairment secondary to altered mandibular mobility, and pain associated with mastication. Larger tumors can also extend into the parapharyngeal space resulting in symptoms associated with pharyngeal compression (Fig. 58-3).
Pleomorphic adenomas generally include a capsule, epithelial and myoepithelial components, along with mesenchymal elements.9 The thickness of the capsule can vary dramatically and the capsule may actually be incomplete in discrete areas of the tumor. Because of this, the extent of required surgical resection is still a matter of significant debate and will be addressed later in this chapter. A wide variety of epithelial cells can be identified including acinar cells; often this component can be hypercellular with a sheet-like pattern. Ducts are often intercalated within more solid epithelial components. Squamous cells can give rise to keratin pearls and spindle-shaped cells can be identified within the myoepithelial component. The bulk of the tumor often consists of the mesenchymal component (which may contain varying amounts of collagen as fibrous tissue, cartilage, even bone). Within the interface between the epithelial and mesenchymal components cystic degeneration may be observed along with squamous metaplasia.9 Although PAs are benign tumors, they may recur, posing significant challenges for the patient and the surgeon. To some degree, recurrence may be a function of both tumor characteristics and manner of treatment. Park et al11 and Malard et al12 both identified satellite tumors as a risk factor for recurrence although Orita et al13 failed to identify this link. The role of capsular rupture in recurrence remains unclear and has led to controversies regarding the precise nature of surgical intervention. Natvig et al14 were unable to identify increased recurrence rates in the case of capsular violation. Herinksson et al found an intraoperative capsular rupture rate of approximately 10% but a similar rate of recurrence for patients with or without capsular rupture; pseudopodia were however found to be associated with higher recurrence (Fig. 58-8A).
Warthin’s tumor are often cystic lesions with a high degree of heterogeneity. The tumor generally is encapsulated. Mucous metaplasia and layers of oncocytic cells can be easily identified. The stromal component is rich in lymphoid tissue and germinal centers can often be recognized. Extensive necrosis is not unusual. Extracellular matrix in the form of fibrous collagen can be seen at the periphery. The overall histologic appearance of WT is relatively specific.9 It does share some characteristics with lymphoepithelial cysts, lymphoma, and lymphoid hyperplasia, but the differential diagnosis can be generally narrowed by a detailed patient history. In cases of massive necrosis, the histology can resemble malignant lesions such as mucoepidermoid carcinoma (MEC). WT can be multifocal and bilateral. Malignant degeneration is possible but uncommon in these lesions; the malignant component can include a wide variety of SG malignant histologies as lymphomas.6,9
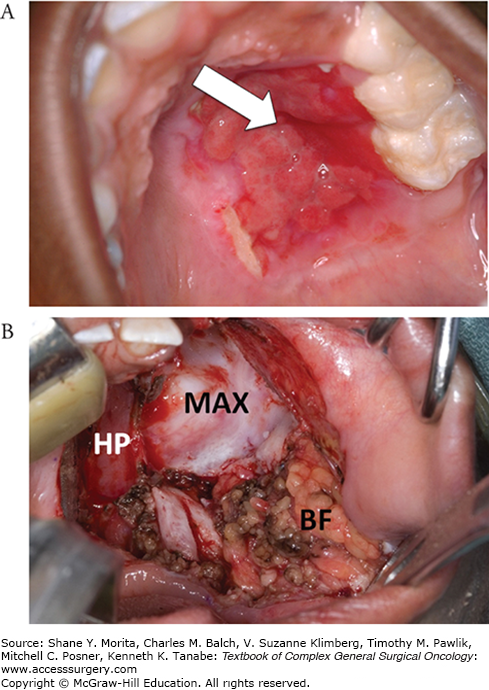
Treatment for benign salivary gland tumors consists primarily of surgical excision. Since these tumors are most commonly encountered in the parotid and SMGs, excision consists of a partial or total parotidectomy or an SMG resection (Fig. 58-4). In cases where the lesion arises from a minor salivary gland, the operation must be tailored to the tumor location and can range from wide local excision of soft tissue to resections including partial maxillectomy for tumors which involve the hard palate (Fig. 58-5).
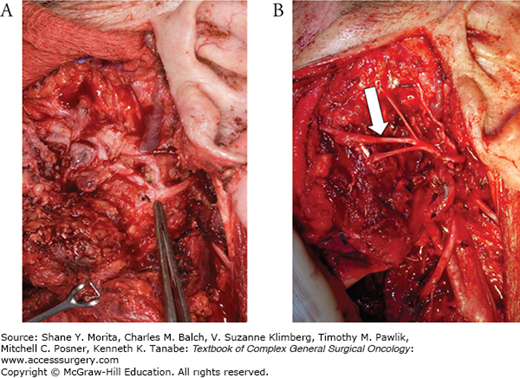
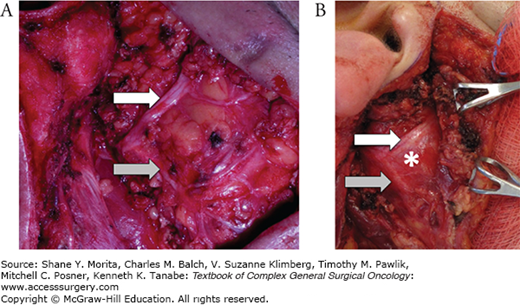
FIGURE 58-4
A. Superficial parotidectomy. The skin flap has been raised and retracted medially (right). A superficial parotidectomy has been performed and the facial nerve has been dissected and preserved. The white arrow points to the superior division of the facial nerve, the gray arrow points to the lower division. B. A deep lobe parotid tumor (asterisk) can be visualized between the upper division (white arrow) and the lower division (gray arrow) of the facial nerve. The superficial lobe of the parotid is retracted medially (right).
In the case of benign tumors, particularly PA, the basic surgical principle is that of complete resection. The greatest controversy surrounds the appropriate management of parotid tumors. This is in large part driven by the morbidity associated with parotid surgery (in comparison with SMG excision, which in most cases can be accomplished by experienced surgeons with minimal morbidity). Attempts to minimize the risk of injury to the facial nerve have led to varying approaches to resection of PA of the parotid gland. Initial attempts at enucleation in the first half of the last century were associated with high rates of recurrence (up to 45%). Although this should not pose an insurmountable problem (because PAs are benign), surgical treatment for recurrent PA is problematic. First, each operation carries with it associated cosmetic concerns which are not trivial. Second, spillage of tumor and seeding of the surgical bed can result in multifocal disease that is difficult to address and greatly increases the risk to the facial nerve in subsequent dissections.15,16
Witt et al found that some capsular exposure is common irrespective of surgical technique and did not appear to increase the rate of recurrence.17 Their accompanying meta-analysis of the literature indicated that enucleation resulted in a nine times higher rate of recurrence compared to superficial parotidectomy (SP). All other techniques (superficial parotidectomy, total parotidectomy, and extracapsular dissection) demonstrated rates of recurrence of roughly 3%. For the majority of benign parotid tumors, superficial parotidectomy (or total parotidectomy in the setting of deep lobe tumors) is the standard of care. While some authors have advocated for the use of extracapsular dissection, this approach should only be utilized by surgeons with substantial clinical experience.18–21
Surgical treatment for PA, although simple in general concept, remains complex and sometimes confusing in application. Donovan and Conley22 perhaps best summarized the dilemmas associated with surgical treatment; en bloc resection is described as the a priori goal of surgery, but reality leads most surgeons to perform occasional capsular dissection and even partial enucleation. Each surgeon should develop an appropriate operative technique that reduces recurrence and minimizes patient morbidity.
An additional consideration in the treatment of PA is the role of adjuvant radiation. Although older literature series describe adjuvant radiation as a means of improving locoregional control (LRC), there is little evidence to support this stance. With several surgical options available for resection with a low rate of recurrence (<10%), there is little role for adjuvant radiation.23 The only indication for EBRT is in the multiply-recurrent setting, where selective use of EBRT may minimize recurrence. However, this approach should be utilized only at experienced centers for highly selected patients.24,25
Treatment for other benign salivary gland tumors resembles that for PA. In general, complete excision of the tumor is favored over enucleation. Adjuvant EBRT is rarely employed, and clinical outcomes are generally good, with acceptable long-term rates of recurrence in the hands of an experienced head and neck surgeon (generally less than 10% for myoepithelioma, basal cell adenoma, WT, cystadenoma).
Clinical outcomes of patients with benign salivary gland tumors are excellent, as described above. Survival is rarely affected by the disease. Locoregional control is adequate with surgery alone. Salivary gland tumors are best addressed by surgeons with extensive experience in head and neck anatomy and pathology in order to minimize peri- and intraoperative risks. Although permanent loss of facial nerve function following surgery is rare, the surgeon must counsel all patients appropriately and be prepared to address surgical complications related to the facial nerve. Other complications associated with parotidectomy include sialocele, Frey syndrome, and salivary fistula.26 Formation of a sialocele following parotidectomy is a relatively common postoperative complication, with reported incidence rates ranging from 6% to 40%.27–30 Resolution is common, and intervention is generally limited to aspiration, although sclerotherapy has also been described by some authors. Frey syndrome results from aberrant superficial reinnervation, resulting in gustatory sweating. Reported incidence rates vary widely and can be as high as 40%.31,32 Frey syndrome rates can be modified by intraoperative interventions such as interpositions of autogenous or allograft material, or rotational muscle flaps.33,34 Nonsurgical treatment can include use of botulinum toxin,35–38 but most patients find the condition tolerable. Botulinum toxin is also often included into the treatment of postoperative salivary fistula.39
Due to the low prevalence and incidence of malignant salivary gland tumors, there have been few randomized controlled trials evaluating current clinical paradigms.40 As such, the vast majority of the literature consists of either large national database studies (i.e., SEER) which are plagued by limited patient and treatment information, or single institution series. Two additional problems limit the quality of existing data on the clinical course and treatment of malignant salivary gland tumors. First, many studies analyze data from varying histologies together, despite substantial evidence demonstrating that histology is one of the key drivers of clinical outcomes. Second, treatment heterogeneity limits the ability to determine whether clinical outcomes are altered by disease characteristics or by treatment selection.
Salivary gland malignancies have been estimated to represent 5% of all head and neck tumors and less than 1% of all malignancies. Although there are 24 different histologic variants, the most common malignant histologies are MEC, adenoid cystic carcinoma (ADC), acinic cell carcinoma(AciC), and carcinoma ex pleomorphic adenoma (CA exPA).9 A SEER database analysis of major salivary gland tumors diagnosed between 1992 and 2006 identified squamous cell carcinoma (SCCA) and MEC as the most common histologies in men and MEC and AciC in women.41 Neither MEC nor ADC demonstrated racial variability. ADC occurred equally in the SMG and parotid, while other histologies had lower incidence rates in the SMG. Overall incidence remained stable over the study period. A single institution study of malignant salivary gland tumors in a Chinese population identified MEC and ADC as most frequently encountered malignant lesions. Interestingly, salivary duct carcinoma (SDC) represented almost 10% of malignant lesions.10 Although the precise population distribution of the malignancies may vary across countries, subpopulations, and institutions, MEC, AciC, ADC, and CA exPA represent the most common malignant histologies of the major salivary glands.
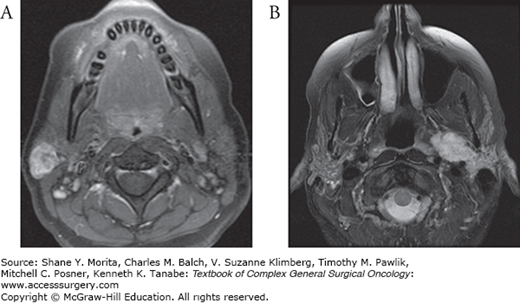
Whereas benign tumors are often encapsulated and have “pushing borders,” malignant salivary gland tumors are poorly defined and exhibit both gross and microscopic invasion of surrounding normal tissue. In the parotid gland, malignant tumors can invade and compress the facial nerve. In fact, facial nerve weakness is a clinical indicator of malignancy in the presence of an uncharacterized parotid mass.42 Perineural spread can involve the facial nerve and extend in a retrograde fashion into the temporal bone. The lack of a defined capsule allows malignant tumors to extend outside the salivary gland itself and violate both deep and more superficial fascial planes (Fig. 58-6). For parotid cancers this can result in invasion of the parapharyngeal space, infratemporal fossa, and orbit. Superficial extension can compromise the soft tissue envelope, significantly complicating reconstructive requirements after oncologic resection. Malignant tumors of the minor salivary glands may pose the most complex surgical challenges. Resection may entail composite resection, partial maxillectomy, partial rhinectomy or in severe cases even craniofacial resections.
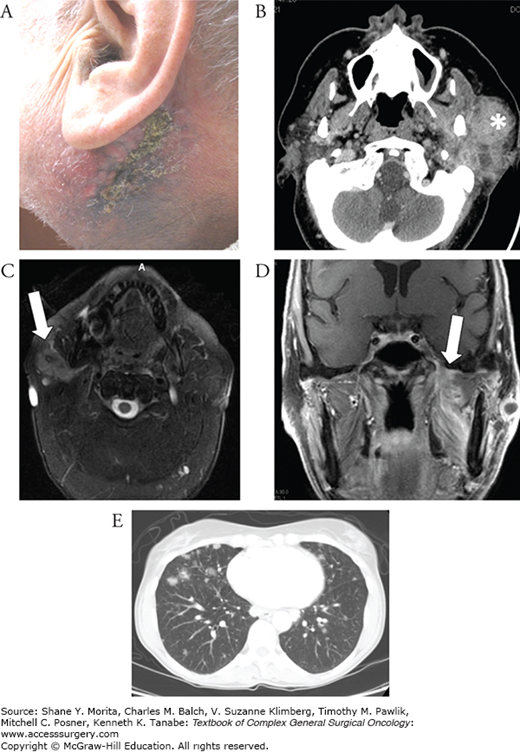
FIGURE 58-6
A. Malignant parotid tumor with skin involvement prior to treatment. B. Contrasted CT scan demonstrating a large malignant (salivary duct carcinoma) parotid tumor (asterisk) encompassing the deep and superficial lobes of the parotid with extension into the parapharyngeal space. C. Contrasted T1-weighted MRI demonstrating a mucoepidermoid carcinoma involving the deep and superficial lobes of the parotid gland. D. Contrasted T1-weighted MRI demonstrating gross perineural invasion through foramen ovale (arrow). E. Contrasted chest CT demonstrating multiple pulmonary metastases from an adenoid cystic carcinoma.
Unlike their benign counterparts, malignant SG tumors have a clinically significant rate of locoregional and distant metastasis (DM) (Fig. 58-6). Since both of these factors as well as clinical outcomes are greatly influenced by histology and tumor grade, additional details are provided in subsequent sections for the more common histologies. Staging for malignant SG tumors is standardized and mirrors the staging pattern for UAEDT SCCA (see Table 58-1). Although tumor grade and histology are not included in the formal TNM staging, they play important roles in treatment selection and clinical outcomes. Low-grade tumors include AciC, epithelial-myoepithelial carcinoma, polymorphous low-grade carcinoma, and basal cell adenocarcinoma. High-grade tumors include solid-type adenoid cystic carcinoma, SDC, anaplastic small cell carcinoma, and carcinosarcoma. Some tumors such as MEC include low-, intermediate-, and high-grade variants.
T Stage
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor ≤2 cm, without extraparenchymal extension |
| T2 | Tumor >2 cm but ≤4 cm, without extraparenchymal extension |
| T3 | Tumor >4 cm or extraparenchymal extension |
| T4a | Tumor invades skin, mandible, external auditory canal, or facial nerve |
| T4b | Tumor invades the skull base, pterygoid plates, and/or carotid artery |
Surgery is the mainstay of treatment for SG cancer, with adjuvant radiotherapy used in select circumstances (see below).
Cancers involving the parotid gland will require a parotidectomy. The extent of the resection is informed by tumor histology, size, pattern of invasion, and the presence or absence of locoregional metastasis. All cancers can be initially approached through a modified Blair incision (which can be extended to a cervical crease in order to allow for an appropriate unilateral neck dissection in cases of either advanced tumor stage/high grade or in the case of demonstrable locoregional metastasis to cervical lymph nodes). Although many surgeons regularly transect the greater auricular nerve during parotidectomy, this is not a necessity, unless there is tumor invading the parotid parenchyma in proximity to the nerve. Vieira et al randomized patients to two groups based on sacrifice of the nerve. Both arms reported transient postoperative numbness, but for patients in whom the nerve was sacrificed, recovery was only partial compared to those patients in which the nerve was preserved.43 A skin flap is usually raised to expose the entire extent of the tumor in the anterior-posterior and superior-inferior dimensions with care to provide sufficient exposure to ensure that adequate margins can be obtained. The thickness of the flap is dictated in part by the tumor and partly by surgeon preference. It may include skin only, skin and subcutaneous fat or skin, subcutaneous fat and superficial musculoaponeurotic system (SMAS). Aggressive large tumors which involve and/or compromise the skin may require excision en bloc with the overlying skin, which will necessitate additional reconstructive techniques in order to close the surgical defect.
Resection of small malignant tumors which are limited to the superficial lobe of the parotid generally involves identification of the facial nerve main trunk using the traditional landmarks of posterior belly of the digastric, tragal pointer, and tympanomastoid suture line. Once the main trunk is identified, it is traced anteriorly to the pez anserinus; upper and lower divisions can be traced anteriorly and individual branches of the nerve identified. If the tumor is clearly confined to the lower portion of the parotid gland, the surgeon may choose to not dissect the upper division branches of the facial nerve if adequate margins can be obtained with a more limited dissection. Minimizing manipulation of the facial nerve reduces the risk of transient or permanent postoperative functional impairment. If the tumor arises from the deep lobe of the parotid, the facial nerve branches should be preserved insofar as possible without violating the principles of oncologic resection and adequate margins.
One of the most controversial areas of salivary gland disease relates to the issue of facial nerve management during surgery. In general, the decision to resect the nerve is not based upon histology, but rather the intraoperative assessment of direct nerve invasion. Preoperative facial nerve weakness should alert the surgeon to possible need for facial nerve resection and reconstruction. Patients with salivary gland cancers should be counseled preoperatively regarding the potential need for facial nerve resection and reconstruction. Branches which are directly involved with the tumor must be sacrificed and the remaining portions (distal and proximal) should be clearly marked and identified. This is particularly important for tumors with a high rate of perineural spread. When clinical evidence for nerve involvement is present, a biopsy of the perineurium can be analyzed by frozen section to determine if histological evidence of invasion is present. Many surgeons send nerve margins along with the main specimen for frozen section analysis in the case of frank nerve invasion. Local control is maximized with complete resection and by obtaining a clear margin along branches of the facial nerve. In the case of large tumors that invade or surround the nerve, it may be necessary to sacrifice most or all facial nerve branches in order to ensure a sound oncologic resection. In this case, the main trunk itself can be evaluated by frozen section to obtain tumor-free margins (Fig. 58-7).
Gross perineural spread can be addressed at the time of initial resection by extending the dissection of the facial nerve posteriorly toward the stylomastoid foramen and if necessary into the mastoid portion of the temporal bone. The impact of surgical technique on disease-related outcomes is difficult to ascertain, in view of the rarity of the lesions themselves. In general, surgeons will tailor the procedure based on tumor characteristics and balance intraoperative risks with the clinical risks of the specific lesion. Chen et al found that whereas most patients underwent superficial parotidectomy for low-grade MEC (160/269) and intermediate grade (274/494), more patients underwent total parotidectomy for high-grade MEC (205/319). Fifty-six percent of patients with high-grade MEC experienced facial nerve sacrifice compared to 28% with low-grade tumors.44
Postresection reconstruction is determined by the extent of the ablative procedure, and patient and surgeon preference. The majority of superficial parotidectomy defects need no reconstruction, although some have advocated the use of a pedicled sternocleidomastoid musculofascial flap for facial contouring.45–47 This is generally only advocated for patients with benign lesions, as rotational flaps may obscure the development of a local recurrence. Small skin defects can be addressed with local reconstruction such as a cervicofacial advancement flap. Large soft tissue defects, which encompass the entirety of the parotid gland and overlying skin often necessitate microvascular soft-tissue transfer to reconstruct both the soft tissue envelope and facial contour. If the facial nerve is sacrificed, local reconstruction may be combined with facial nerve grafting and local static techniques, including upper eyelid gold weight, static slings for the oral commissure, and lateral tarsorrhaphy in order to improve both cosmesis and function of the eye and mouth. A thorough discussion of management of facial nerve paralysis is beyond the scope of this chapter.
Neck dissection for regional disease should be tailored to preoperative imaging, surgeon experience, and primary histology. Therapeutic node dissection for SC cancers generally entails levels I–V. Substantial controversy surrounds the indications for elective neck dissection and the role of neck dissection in achieving LRC. Lau et al found that among 119 clinically N0 tumors, 21% had pathologically proven disease, with the highest incidence noted among patients with adenocarcinoma and high-grade MEC. The rate of occult disease was as high as 33% for T1–2 lesions and 38% for T3–4 disease. Subclinical nodal metastases were most commonly identified in level II followed by level III. Occult disease for levels I, IV, and V was ≤5%.48 Chen et al49 found that most nodal recurrence following definitive treatment occurred in levels I or II.
Zbaren et al compared patients who underwent elective node dissection (END) to those who were observed in a cohort of 83 previously untreated parotid tumors (of mixed histology). Among patients who underwent END, eight occult cervical metastases were detected, of which five were present in patients with high-grade tumors. Recurrences occurred in 12% of patients in the END group and 26% of patients in the observation group, with all neck recurrences occurring in the observation group.50 Armstrong et al51 identified size and grade as risk factors for occult regional metastasis. Tumors ≥4 cm had a 20% risk of occult metastasis compared with 4% for tumors <4 cm. The impact of grade was maintained irrespective of tumor histology. Ettl et al52 identified advanced age, advanced T stage, high grade, and specific histologies (salivary duct) as distinct risk factors for metastasis to neck nodes.
In the absence of randomized controlled trials, which control for tumor histology, the role of elective neck dissection in the treatment of salivary gland malignancies remains unclear. It appears safe to conclude from existing retrospective series that patients with large or high-grade tumors could benefit from elective neck treatment. However it remains unclear precisely what impact elective neck treatment has on patient survival. Given the morbidity associated with neck dissection, it is important to define the patients who are at high risk of occult locoregional spread and could presumably benefit from END. Based on these and other similar studies, END is generally considered in the preoperative planning for patients with advanced stage and high-grade tumors.53 One consideration is performing intraoperative biopsies of suspicious-appearing nodes in the primary echelon when the indications for END are equivocal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree