Tumors of the Parathyroid Gland
Katrina Chaung
William S. Duke
David J. Terris
The parathyroid glands maintain a delicate calcium homeostasis within the body, and dysfunction of these glands may result in significant sequelae. The recognition and treatment of parathyroid disorders can be challenging. Modern advances in imaging techniques and intraoperative parathyroid hormone (PTH) assays are invaluable in the contemporary management of parathyroid disorders.
HISTORY
The history of the parathyroid glands, from their discovery to the understanding of their physiologic and pathologic importance, unfolded somewhat recently. In November of 1849, the two-ton great Indian Rhinoceros owned by the Zoological Society of London passed away. Sir Richard Owen, a renowned British anatomist and Conservator of the Museum in the Royal College of Surgeons, dissected the cadaver in his own resident quarters.1,2 He provided the first recorded description of the parathyroid gland: “a small compact yellow glandular body attached to the thyroid at the point where the vein emerged.”1
In 1880, a Swedish medical student Ivar Viktor Sandström identified a vascularized organ distinct from the thyroid gland and lymph nodes that he named glandulae parathyroidae.1,2,3 In his writings, Sandström even noted the variable location of these entities.3
It was not until the work of French physiologist Eugene Gley some years later that the function of these structures became evident.1 Tetany was first described following thyroidectomy cases performed by Bilroth in the late 1870s. Initially, this complication was attributed to hyperemia of the brain as a result of the thyroidectomy.3 Gley observed that tetany and death ensued if these glandulae parathyroidae were excised during thyroidectomy in dogs. Further studies revealed that the abnormal neuromuscular sequelae seen in animals postthyroidectomy could be prevented by autotransplantation of these glands.1
In 1891, Friedrich Daniel von Recklinghausen described lesions that he termed osteitis fibrosa cystica of von Recklinghausen.1,2 A Viennese pathologist, Jakob Erdheim, also found enlarged parathyroid glands on autopsy of patients with osteomalacia and osteitis fibrosa cystica.3 At that time, the bone disease was not attributed to a parathyroid abnormality; in fact, parathyroid enlargement was believed to be a compensatory consequence of the bone disease.1,2,3 In 1915, another Viennese pathologist, Friedrich Schlagenhaufer, described two patients with osteomalacia who were each found to have a single parathyroid tumor at autopsy, suggesting that the enlarged parathyroid glands might actually be the cause of the bone disease.1
It was not until 10 years later that Felix Mandl, a Viennese surgeon, attempted the first excision of a parathyroid tumor in a patient with severe von Recklinghausen disease. The patient, a tram conductor, was severely disabled and unable to ambulate due to his disease.1,2 He underwent a neck exploration with excision of a 25-mm “yellowish-brown almond-shaped tumor” in the area of the left inferior thyroid gland. A few days postoperatively, the patient was able to walk without assistance and demonstrated significantly lower blood and urine calcium levels. He did well for 6 years before experiencing recurrent hypercalcemia and nephrolithiasis. Upon reoperation, Mandl was unable to find abnormal parathyroid tissue, and the patient passed away shortly thereafter. The recurrence was attributed to ectopic parathyroid tissue, but, interestingly, none was found at autopsy.1,2
The first parathyroid operation in the United States followed shortly thereafter. The patient, sea captain Charles Martel, was diagnosed with hyperparathyroidism (HPT) and had lost 6 inches in height due to associated bone demineralization.2,3 He underwent six operations, beginning in 1927, at the Massachusetts General Hospital that either removed normal parathyroid tissue or found no abnormal tissue. By 1932, his renal function had begun to deteriorate and Dr. Churchill, under the insistence of Martel himself, planned a mediastinal exploration to search for ectopic parathyroid tissue. A 3-cm tumor was identified in the mediastinum and removed. Unfortunately, the patient developed an impacted ureteral stone several weeks later and passed away from laryngospasm during a procedure to relieve the obstruction.2 The first documented parathyroid carcinoma was excised at Cook County Hospital in Chicago in 1926. The patient, a 29-year-old female, had multiple masses in the neck and required multiple subsequent excisions for recurrences.3
In 1963, Berson and Yalow developed a radioimmunoassay for PTH that allowed for the accurate acquisition of serum PTH levels.2,3,4 The development of multichannel autoanalyzing systems made it possible to rapidly determine serum calcium levels. With the advent and subsequent improvement of these innovations, asymptomatic patients could be diagnosed with parathyroid abnormalities before presenting with advanced bone and renal disease, allowing for more expeditious and efficacious management.
EMBRYOLOGY AND ANATOMY
During the 5th week of gestation, the inferior and superior parathyroid glands develop from the dorsal endoderm of the third and fourth branchial pouches, respectively.2,4,5 As the cervical spine lengthens and the heart and great vessels descend, the inferior glands migrate caudally within the tail of the thymus. As the thymus migrates further into the upper thorax, its tail involutes, leaving the inferior glands near the inferior
poles of the thyroid gland.2,4,5 Concurrently, the superior glands migrate intimately associated with the ultimobranchial bodies, halting at the lateral aspect of the posterior superior to the middle third of the thyroid gland. This disparity in migrational distance during development explains the increased variability of anatomic locations of the inferior parathyroid glands as compared to those of the superior glands.5 Ectopic parathyroid glands have a reported incidence ranging from 6% to 19%.6,7
poles of the thyroid gland.2,4,5 Concurrently, the superior glands migrate intimately associated with the ultimobranchial bodies, halting at the lateral aspect of the posterior superior to the middle third of the thyroid gland. This disparity in migrational distance during development explains the increased variability of anatomic locations of the inferior parathyroid glands as compared to those of the superior glands.5 Ectopic parathyroid glands have a reported incidence ranging from 6% to 19%.6,7
Most individuals have four parathyroid glands. Fewer than four glands have been found in ˜2% to 3% of individuals.8,9,10,11 The incidence of supernumerary glands ranges from 5% to 13%.5,8,9 Dissection of 428 cadavers by Gilmour12 in 1928 revealed 5 parathyroid glands in 6.7% of the specimen, 6 in 0.05% of the specimen, and 12 parathyroid glands in one specimen. Supernumerary glands are commonly found in the mediastinum within the thymus or associated with the thyrothymic tract.2 Surgeons must be cognizant of the potential variability in the number of parathyroid glands, particularly in patients with multigland disease. In one study of over 2,000 patients who underwent surgery for primary HPT, the offending parathyroid adenoma was found to be a supernumerary gland in 0.7% of the cases.13
The superior parathyroid glands lie dorsal to the coronal plane created by the course of the recurrent laryngeal nerve (RLN) through the neck; the inferior glands lie ventral to this plane.5 This relationship to the plane of the RLN is nearly always constant and therefore an important concept during surgical exploration of the glands.
In approximately 80% of patients, the superior parathyroid glands can be identified within 1 cm above the intersection of the RLN and inferior thyroid artery. They are usually intimately associated with the thyroid capsule at the posterosuperior thyroid pole.2,5 The glands can be distinguished from neighboring thyroid nodules by their mobility within the extension of the pretracheal fascia that also envelopes the thyroid gland.2 Common locations of ectopic superior parathyroid glands include paraesophageal, retroesophageal, and retrolaryngeal sites; the tracheoesophageal groove; within the thyroid gland; the posterior mediastinum; and within the carotid sheath.5,7,14 In rare instances, they are found in lateropharyngeal or intercricothyroid and retropharyngeal locations.2 In some instances, the superior glands may be overly descended, lying inferior to the inferior parathyroid glands; alternately, they may also be found in an undescended location near the hyoid bone or pharyngeal musculature.4
The inferior parathyroid glands are generally found on the posterolateral aspect of the lower thyroid pole, inferior to the intersection of the RLN and inferior thyroid artery. They may also be found within the thyrothymic ligament.7,15 As mentioned previously, due to its longer course of migration during development, the inferior glands have more variability in anatomic position than the superior glands.5 Additionally, if there is a delay or failure to separate from the thymus, the inferior glands may be drawn into the anterior mediastinum.5 Common ectopic locations include the thyrothymic ligament and anterior mediastinal, intrathymic, and intrathyroidal positions.5 Less common ectopic sites include the aortopulmonary window, the carotid sheath, and pericardial and pyriform sinus locations.2,7
Acquired ectopia of enlarged neoplastic and normal parathyroid glands results from migration under the influence of gravity, changes in intrathoracic pressure, deglutition, and mechanical mass effect as in the case of large goiters. In such instances, superior parathyroid glands can migrate along the posterior vertebral fascia into the posterosuperior mediastinum.2,7,16 Similarly, the inferior glands can migrate into the anterior mediastinum along the thyrothymic ligament.5 It is estimated that up to one-third of missed parathyroid adenomas are ultimately located in the anterior mediastinum.2
With some variability, the superior glands derive their blood supply from a single branch off of the inferior thyroid artery. Occasionally, the arterial supply is from a branch of the superior thyroid artery or from an arterial anastomoses.2,5 Venous drainage is usually via the superior or lateral thyroid veins. The inferior thyroid artery also supplies the inferior parathyroid glands; this relationship is often retained even in cases of ectopic mediastinal migration, but rare cases of arterial supply from a branch of the internal mammary artery or a direct branch off the aortic arch have been reported.5,15 Venous drainage is often via the lateral or inferior thyroid veins.5 Lymphatic drainage of the parathyroid glands parallels that of the thyroid gland, draining into the paratracheal and deep cervical lymphatic nodes.2 Preservation of the vascular supply during surgery is crucial in maintaining the viability and function of the gland.
Normal parathyroid glands are smooth with an oval or bean shape. They typically weigh between 40 and 50 mg, though some studies have cited the upper limit of normal to be 65 mg.2,5 Variations in weight have been observed between ethnicities, genders, comorbidities, and even within individuals. Contingent upon vascularity, adipose tissue, and oxyphil content, their color ranges from tan to reddish brown.2,4 Parathyroid glands can easily be confused with adipose tissue, thyroid nodules, lymph nodes, or thymic remnant. They may be distinguished from adipose tissue by a characteristic “gliding” motion against the surrounding tissues. Careful visual observation and subtle differences in palpation can distinguish between a normal and abnormal gland.
Histologically, a thin connective tissue capsule is present with septae extending into the parenchyma, dividing the gland into clusters of functional cells. The parenchyma is predominantly composed of chief cells residing in an adipose tissue-rich stroma. The functional chief cells are slightly eosinophilic and contain copious PTH secretory granules. The percentage of adipose tissue content in normal glands, previously thought to be a marker of functional status, has been seen to vary among individuals depending on age, gender, and possibly other factors.2 Oxyphil cells contain an abundance of mitochondria. Although their function is unknown, some theorize that their high mitochondrial content may explain the high avidity of abnormal parathyroid glands for the technetium (Tc)-99m used in localization scans.2
PHYSIOLOGY
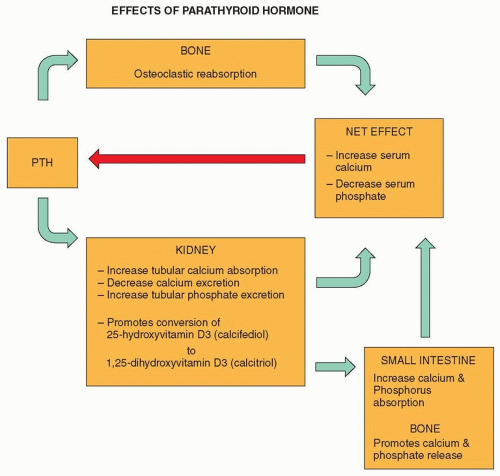
PTH is an essential factor in the intricate balance of serum calcium homeostasis. PTH secretion is primarily in response to low serum ionized calcium levels, the active component of serum calcium. This is a sensitive relationship; even minute alterations in calcium concentration by as little as 0.04 mmol/L have been shown to elevate serum PTH by 100% or more.2 Its influence is exerted via cyclic adenosine monophosphate (cAMP) pathways on bone to increase osteoclastic
reabsorption and on the kidneys to increase tubular calcium absorption, decrease calcium excretion, and increase tubular phosphate excretion. Additionally, PTH promotes renal 1α-hydroxylase-mediated conversion of 25-hydroxyvitamin D3 (calcifediol) to 1,25-dihydroxyvitamin D3 (calcitriol). This biologically active form of vitamin D, in turn, stimulates the small intestine to increase absorption of calcium and phosphorus. Calcitriol also promotes the release of calcium and phosphate from bone. The net effect is an increase in serum calcium and a drop in serum phosphate. The increase in serum calcium produces negative feedback to decrease the secretion of PTH (Fig. 20.1). As a further feedback mechanism, if calcium levels are too high, calcitonin, produced by parafollicular C cells of the thyroid gland, inhibits calcium reabsorption from bone and increases renal clearance of calcium and phosphate.2,5,17
reabsorption and on the kidneys to increase tubular calcium absorption, decrease calcium excretion, and increase tubular phosphate excretion. Additionally, PTH promotes renal 1α-hydroxylase-mediated conversion of 25-hydroxyvitamin D3 (calcifediol) to 1,25-dihydroxyvitamin D3 (calcitriol). This biologically active form of vitamin D, in turn, stimulates the small intestine to increase absorption of calcium and phosphorus. Calcitriol also promotes the release of calcium and phosphate from bone. The net effect is an increase in serum calcium and a drop in serum phosphate. The increase in serum calcium produces negative feedback to decrease the secretion of PTH (Fig. 20.1). As a further feedback mechanism, if calcium levels are too high, calcitonin, produced by parafollicular C cells of the thyroid gland, inhibits calcium reabsorption from bone and increases renal clearance of calcium and phosphate.2,5,17
Once secreted from the parathyroid gland, the biologically active (1 to 84) PTH molecule is rapidly degraded, with a half-life of 3 to 5 minutes in individuals with normal renal function. The intact form is cleaved into a biologically active aminoterminal fragment that is rapidly cleared from the circulation and an inactive carboxyl-terminal fragment that undergoes slow renal clearance.2,17 The initial PTH radioimmunoassays took up to 24 hours to obtain results. With the development of immunoradiometric assays and subsequent immunochemiluminometric assays (which utilize distinct antibodies for the amino and carboxyl terminals of the intact PTH molecule), the process can be reduced to <15 minutes. Newer assays that measure the intact hormone are a more accurate measure as compared to older assays that principally measured the inactive fraction.2,17 Such assays can be used for rapid intraoperative assessments of PTH levels. Recent studies have found that some assays may utilize antibodies that cross-react with fragments they were not intended to target. This may hold especially true for patients with renal failure and may account for some of the variability between different assays types.17
BENIGN LESIONS
Parathyroid Adenoma
Parathyroid adenomas are benign tumors of the parathyroid gland and account for 80% to 90% of primary cases of HPT.2,5 Parathyroid adenomas have a female predominance with an increased incidence in individuals over the age of 50. An association between head and neck ionizing radiation exposure at an early age and development of parathyroid adenomas has also been shown.19,20 The majority of adenomas occur sporadically and usually involve a solitary gland. Some sources indicate inferior gland involvement more frequently than the superior glands.2,5,21 The incidence of multiple adenomas has been reported to range from 1.7% to 11.5%.22,23
Parathyroid adenomas are monoclonal neoplasms composed of a clonal expansion of cells with altered calcium sensitivity.22,24 Various genomic events have been proposed as the trigger for this cellular propagation. Among the events
recognized are a rearrangement of protooncogene parathyroid adenomatosis 1 (PRAD1) into the vicinity of the regulatory region responsible for PTH synthesis.25,26 Somatic mutations of the multiple endocrine neoplasia 1 (MEN1) tumor suppressor gene have been demonstrated in 20% of primary HPT cases.27 The loss of tumor suppressor function, specifically of loci, located on chromosome 1p, is believed to be an even more common cause of sporadic adenomas.28
recognized are a rearrangement of protooncogene parathyroid adenomatosis 1 (PRAD1) into the vicinity of the regulatory region responsible for PTH synthesis.25,26 Somatic mutations of the multiple endocrine neoplasia 1 (MEN1) tumor suppressor gene have been demonstrated in 20% of primary HPT cases.27 The loss of tumor suppressor function, specifically of loci, located on chromosome 1p, is believed to be an even more common cause of sporadic adenomas.28
Upon gross examination, parathyroid adenomas tend to have a more reddish-brown coloration and an increased, rubbery consistency as compared to normal glands. Adenomatous glands may be bi- or multilobed with a smooth, nodular, or cysticappearing capsule.2 Histologic examination reveals hypercellular nodules of chief cells arranged in sheets or cords surrounded by a rim of normal-appearing parathyroid tissue. Compared with those contained in normal glands, chief cells within adenomas are larger with more nuclear pleomorphism and giant cell formation. Nuclear atypia and mitotic figures may also be observed, but these characteristics are not of diagnostic value in distinguishing between benign or malignant parathyroid abnormalities.2,5,29
Various subtypes of parathyroid adenomas have been reported, including oncocytic adenomas, lipoadenomas, water-clear cell adenomas, large clear cell adenomas, and atypical adenomas. Most have previously been thought to be nonfunctioning entities; however, recent evidence may suggest associations with HPT.2
Hyperplasia
Primary chief cell hyperplasia causes enlargement of all parathyroid glands either uniformly or variably and accounts for 5% to 15% of primary HPT cases. As with parathyroid adenomas, hyperplasia also shows a female predilection.2,5 It is characterized by a proliferation of chief cells, and sometimes oncocytes, in multiple glands, causing an increase in parenchymal bulk without a known stimulus for elevated PTH secretion. The parenchymal cells can form solid sheets, cords, or follicles.5 Nuclear atypia and mitotic figures are very rarely seen.2 Hyperplastic glands lack the “rim” of normal parathyroid tissue found in adenomas, but a “pseudorim” around nodules may be seen within the hyperplastic glands.5 Approximately 30% of cases are associated with some type of familial HPT or MEN syndrome.2
A rare form of hyperplasia, water-clear cell hyperplasia, can cause significant hypercalcemia. Interestingly, this is the only condition where the superior parathyroid glands are consistently larger than the inferior glands. Glands affected by this hyperplasia are often asymmetrically enlarged with lobular extensions and have a dark chocolate brown color. Microscopically, there is a diffuse proliferation of clear cells with small dense nuclei and cytoplasm filled with small vacuoles.2,5 Histologically, the appearance is similar to that of renal cell carcinoma.2
In cases of hyperplasia secondary to renal failure, the glands appear to be more uniform in size early in the disease. Asymmetry becomes more pronounced as the disease progresses, and the degree of enlargement seems to mirror the severity of renal disease.2
Multigland Disease
The incidence of synchronous double parathyroid adenomas ranges from 2% to 10% and increases with age. It is estimated that ˜50% of double adenomas localize to two distinct locations on preoperative studies.2 Intraoperative PTH assessment can be extremely helpful in reflecting the sequential decrease in hormone levels after excision of each hyperfunctional gland. Identification of at least one normal gland is crucial in ruling out four-gland hyperplasia.
In approximately 10% to 15% of patients with sporadic primary HPT, diffuse hyperplasia of the parathyroid glands may be present.2 A thorough medical and family history must be performed to rule out MEN syndrome. Preoperative localization studies may not localize in these cases. Bilateral exploration should be performed if this condition is suspected. Intraoperative PTH levels are useful to confirm that all hyperfunctional tissues have been removed.
MALIGNANT LESIONS
Parathyroid Carcinoma
Parathyroid carcinoma is a rare malignancy and accounts for <1% of cases of primary HPT. In contrast to the previously mentioned etiologies of primary HPT, there is equal gender distribution, and this condition often presents during the fourth through fifth decades, ˜10 years younger than patients with benign primary HPT. There is no apparent ethnic or geographic predominance.5,30,31 Purported association with history of head and neck irradiation, chronic renal failure, and familial HPT, in particular familial isolated HPT and hereditary hyperparathyroidism-jaw tumor syndrome (HPT-JT) and MEN syndromes, has been reported.30,32,33,34 Overexpression of cyclin D1 has been found in a high percentage of parathyroid carcinomas, but the significance of that finding is unknown. An inactivating mutation of a recently described tumor suppressor gene HRPT2 that encodes for the protein parafibromin, a protein with unknown function, has been identified as a possible factor in the pathogenesis of parathyroid carcinomas.30,35
Parathyroid carcinomas have been identified in eutopic, ectopic, and supernumerary glands.36 In contrast to patients with benign causes of primary HPT who generally present with mild hypercalcemia, patients with parathyroid carcinomas can present with significantly elevated calcium levels that are 3 to 4 mg/dL above the upper limit of normal.5,30 Approximately 70% of patients present with serum calcium levels above 14 mg/dL and intact PTH levels at least five times the upper limit of normal. Additionally, a palpable firm cervical mass is noted in 30% to 50% of patients upon presentation.2,37 Eighty percent of patients complain of hypercalcemic symptoms including fatigue, weakness, and polydipsia, and many manifest evidence of target organ effects, specifically within the kidney and skeletal system. One series reported nephrolithiasis in 56% and renal insufficiency in 84% of patients with parathyroid carcinoma; another cited concomitant bone and renal disease in 50%.30,31,37 This is in contrast to patients with benign causes of HPT where only about half complain of symptoms and neck masses are very rarely seen.30 The finding of RLN palsy should also heighten suspicion for carcinoma.2,5,38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree