Tumors of the Parapharyngeal Space
Zhen Gooi
Matthew J. Kruse
David W. Eisele
Jeremy D. Richmon
The evaluation and treatment of tumors of the parapharyngeal space (PPS) have evolved over the past few decades. Successful management of tumors in this region is now a multidisciplinary endeavor encompassing a broad range of medical and surgical subspecialty knowledge. There is an increasing awareness of the need to balance the benefits of surgical resection with the anticipated functional deficits and subsequent morbidity that can occur following surgical intervention. An intimate understanding of the anatomy of this region remains the key factor in surgical planning and successful management of tumors involving this anatomic region.
ANATOMY
The PPS is a potential space deep in the neck, shaped like an inverted pyramid. The borders delineating this space are made up of both immobile and distensible tissues, which has implications on the directions of growth and eventual clinical presentation of tumors occurring in this space. At its superior limit, representing the base of the inverted pyramid, is a small region of the temporal and sphenoid skull base including the carotid canal, jugular foramen, and hypoglossal foramen. The inferior limit representing the apex is at the level of the greater cornu of the hyoid bone. The medial borders of this space are the pharyngeal constrictor muscles of the oropharynx, a distensible plane that enables tumor expansion. The medial pterygoid muscle, ramus of the mandible, and the deep lobe of the parotid gland represent the lateral borders, whereas the prevertebral fascia and cervical vertebrae make up the posterior border. The superior, lateral, and posterior borders are relatively immobile barriers, and as such, tumor growth typically occurs in either the medial or inferior direction leading larger tumors involving these areas to manifest as a pharyngeal mass or cervical mass.
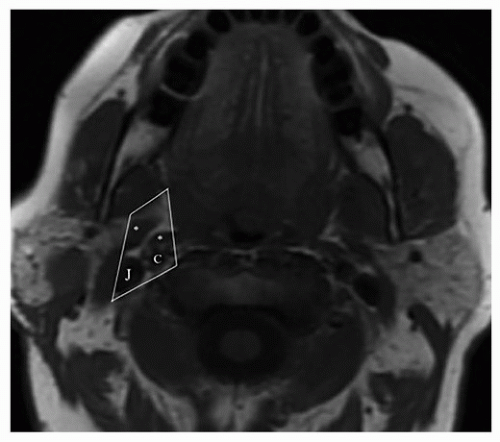
The PPS has traditionally been divided into prestyloid and poststyloid compartments by the fascial band connecting the styloid process and skull base to the tensor veli palatini muscle (Fig. 22.1). The prestyloid space, positioned anterolaterally, is a potential space composed of adipose tissue, lymph nodes, minor salivary glands, branches of the mandibular division of the trigeminal nerve, internal maxillary artery, and the pharyngeal venous plexus. The poststyloid space, positioned posteromedially, contains the carotid artery, internal jugular vein (IJV), cranial nerves IX to XII, the sympathetic chain, and lymph nodes. In relation to other neck spaces, both the masticator and parotid spaces are located anterolaterally, whereas the retropharyngeal space is located posteromedially. Recently, a suggested update to the nomenclature of the PPS refers to the prestyloid space as the true PPS, whereas the poststyloid space is referred to as the vascular space.1
CLINICAL PRESENTATION
The vast majority of patients with a mass in the PPS have little to no symptoms until the size of the mass is sufficiently large. This is due in part to the relatively slow growth of tumors that arise in this area. Therefore, it is not surprising that many of these masses are found as incidental findings on imaging studies performed for other reasons. Nonetheless, there are multiple symptoms that may be related to tumors arising within the PPS reflecting the diverse pathology that can arise from within this region. These can be categorized broadly into symptoms related to tumor mass effect or tumor invasion such as functional deficits from cranial nerve involvement. Natural expansion of tumor along the path of least resistance along the medial boundary leads to displacement of the lateral pharyngeal wall and tonsil that may manifest as dysphagia, dysarthria, newonset snoring, voice changes, or an ill-fitting maxillary denture due to soft palate displacement of the tumor. Tumors narrowing the oropharyngeal region may lead to symptoms of obstructive sleep apnea.2,3 Hearing loss can arise from middle ear effusion by compression of the cartilaginous portion of the eustachian tube, whereas the presence of trismus implies infiltration of the pterygoid musculature or impingement on the coronoid process of the mandible. Tumors arising in the superior portion of the PPS in close proximity to the skull base may cause manifestations of a jugular foramen syndrome characterized by nerve palsies of cranial nerves IX, X, and XI. Extension of tumor along an inferior plane can lead to a palpable cervical mass posterior and inferior to the angle of mandible or tail of the parotid. Involvement of cranial nerves or the cervical sympathetic chain may cause symptoms of dysphonia from vocal cord paralysis or paresis, dysarthria from tongue weakness, or Horner syndrome.
DIAGNOSTIC CONSIDERATIONS
Diagnostic Imaging
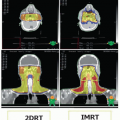
As mentioned above, many PPS masses are discovered incidentally on imaging studies that include the head and neck region. However, the preferred radiologic modality in the assessment of PPS tumors is magnetic resonance imaging (MRI) with gadolinium enhancement as it allows for precise localization of tumors in relation to surrounding neurovascular and muscular structures, thus helping to differentiate between benign and malignant masses. T1 sequences are useful for defining the interaction between the tumor and surrounding fat planes, whereas T2 sequences delineate the relationship between tumor and adjacent muscle. Postcontrast T1 imaging allows for assessment of perineural spread. The use of particular MRI sequences including phase contrast angiography, time of flight,
and MR angiography have been shown to increase the diagnostic sensitivity and specificity of PPS lesions.
and MR angiography have been shown to increase the diagnostic sensitivity and specificity of PPS lesions.
Although MRI remains the preferred modality, newer multidetector CT scanning has a number of advantages including quicker scanning times, improved spatial resolution up to 0.082 mm3 (compared to 4.4 mm3 for T1-weighted images and 1.1 mm3 for time of flight MR angiography), and ability for multiplanar reconstruction of images.4 Quicker scanning times of <30 seconds make images less susceptible to motion artifacts compared to MRI and should be a consideration in patients who are not candidates for MRI or experience claustrophobia. There are considerable cost savings with CT scan compared to MRI as well. Ultrasound is an inexpensive, noninvasive, nonionizing radiation diagnostic modality that has the added advantage of enabling radiologic guidance for fine needle aspiration biopsy (FNAB).
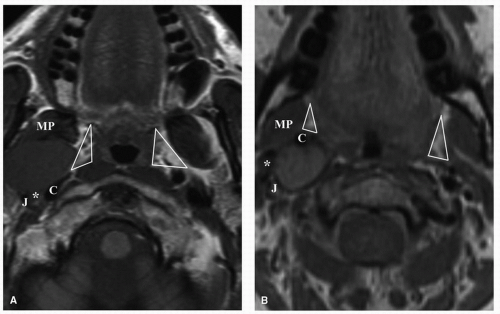
The diagnosis of a tumor within the PPS can be predicted based on a distinction made on its location within either the prestyloid or poststyloid space. The majority of tumors found within the prestyloid compartment are of salivary gland origin, whereas in the poststyloid compartment, tumors of neural origin constitute the majority of lesions. The determination of whether a tumor is located in the prestyloid or poststyloid space is based on a number of factors including tumor position relative to the carotid artery, styloid process, medial pterygoid muscle, and parapharyngeal adipose tissue plane. In prestyloid masses, the carotid artery is posteriorly displaced, the medial pterygoid muscle is anterior in relation to the tumor, and the parapharyngeal adipose tissue plane will be located along its medial aspect. In poststyloid masses, the carotid artery is displaced anteriorly, whereas the parapharyngeal adipose tissue is displaced in an anterolateral direction (Fig. 22.2). As a poststyloid mass expands laterally, it will extend posterior to the styloid process.
Fine Needle Aspiration Biopsy
Proper imaging is usually sufficient to determine the type of tumor in the PPS in most cases. Due to this, the use of FNAB for diagnosis is selective. Imaging studies may help differentiate tumors that require surgical resection from those that do not. Also, the extent of operation is infrequently altered by the FNAB results. Nonetheless, FNAB may be used as a diagnostic adjunct for preoperative evaluation of PPS tumors and to aid in treatment planning and patient counseling, in particular if a malignant diagnosis is suspected or in cases of uncertain radiologic interpretation. A number of case series has shown that FNAB confers a diagnostic accuracy of >87% for malignant lesions.5,6,7,8 The overall diagnostic accuracy of FNAB for all tumor types when compared to histopathologic analysis of resected surgical specimens has ranged from 36% to 61%.5,6,8 A recent study has highlighted a significant improvement in diagnostic accuracy when liquid-based cytology is employed in the place of conventional smear preparations.5 Coordination of care with a cytopathologist at the time of FNAB can help to ensure a satisfactory specimen quality especially in cystic lesions.
COMMON PARAPHARYNGEAL SPACE TUMORS
Salivary Tumors
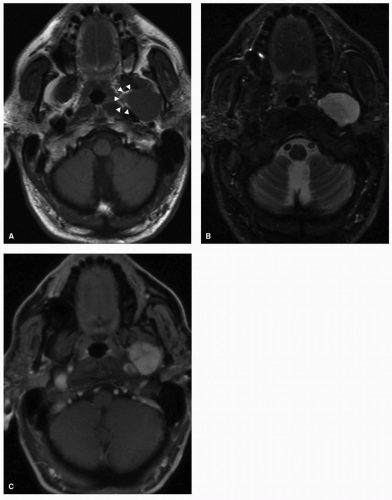
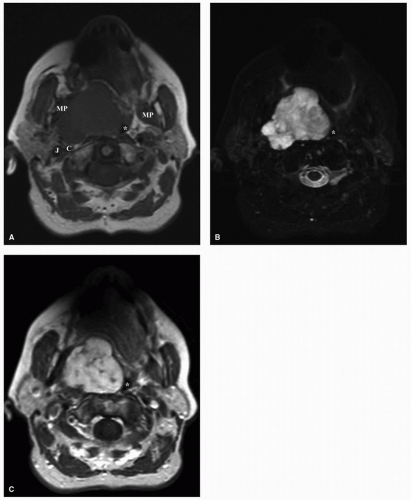
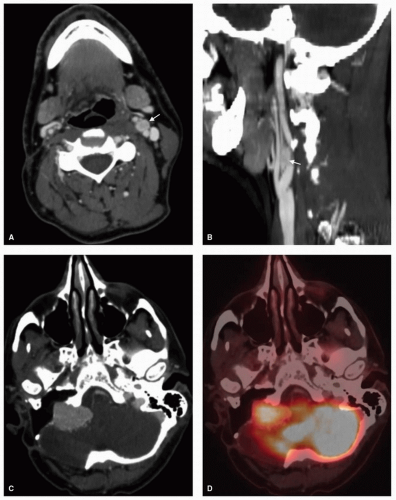
Salivary gland tumors within the PPS can arise from the deep lobe of the parotid gland or from ectopic salivary gland tissue within the PPS itself, and they constitute the most common PPS tumors. Distinguishing between these two entities is not possible on physical examination. Radiologically, deep lobe parotid tumors extending into the PPS are observed to be contiguous with the parotid gland, whereas de novo tumors have a clear plane of separation with the overlying parotid tissue. Consequently, the overlying parotid tissue can be spared in the excision of de novo tumors although this is not often possible for contiguous tumors of the parotid deep lobe. A comprehensive discussion of salivary tumors is covered in Chapter 21. However, here we aim to present the key imaging characteristics of these tumors. Pleomorphic adenomas are the most common tumors arising from the prestyloid PPS.9,10,11 There is a degree of heterogeneity observed on CT and MRI, depending on the size of the tumor with more uniform characteristics observed in smaller tumors. On MRI, smaller tumors are usually well circumscribed with a low-intensity T1 signal that enhances homogenously on postcontrast imaging with a hyperintense T2 signal (Fig. 22.3). Larger tumors are often lobulated and may demonstrate areas of hyperintense or hypointense signal intensity attributed to calcification, necrotic, or hemorrhagic areas within the tumor (Fig. 22.4).12 Malignant degeneration can occur in pleomorphic adenomas giving rise to carcinoma ex pleomorphic adenoma or carcinosarcoma. These malignancies are associated with a high frequency of metastasis to regional lymph nodes and can also metastasize to the lungs, bone, and brain.
Mucoepidermoid carcinoma is the most common malignant tumor of the parotid gland, and imaging findings on MRI vary depending on tumor grade. Lower-grade tumors can exhibit similar features to pleomorphic adenoma with low to intermediate signal on T1 images, intermediate to high signal on T2 imaging correlating with the increased mucin-secreting cellular component, and heterogenous enhancement on postcontrast T1 images. High-grade tumors demonstrate low to intermediate signal on both T1 and T2 images with ill-defined margins.
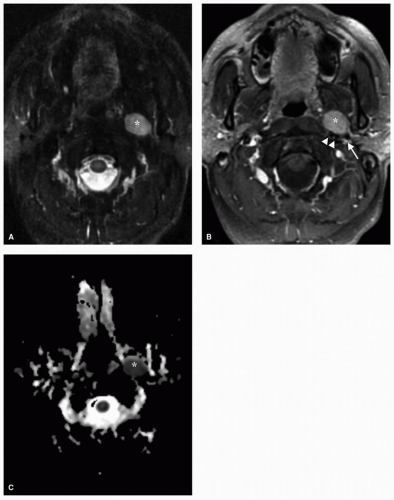
As a general principal, features of salivary gland neoplasms on MRI that have been shown to be indicative of malignancy include low signal intensity on T2-weighted imaging, ill-defined margins on postcontrast images, and perineural involvement. The radiologic features of benign parotid tumors and lowgrade malignancies share similarities on MRI, and definitive diagnosis based on imaging studies alone can be a challenging task. The use of dynamic contrast MRI with analysis of peak contrast enhancement and subsequent time frame of contrast washout can assist in distinguishing between Warthin tumor and malignant lesions.13,14 The comparison of various apparent diffusion coefficient (ADC) of tumors on diffusion-weighted MRI has also been analyzed, with higher ADCs being associated with pleomorphic adenomas and Warthin tumors and lower ADCs associated with malignant tumors (Fig. 22.5).15,16 The use of fluorodeoxyglucose positron emission tomography (FDG PET) for the diagnosis of salivary gland tumors is limited owing to the nonspecific nature of FDG uptake, with some benign tumors such as Warthin tumor demonstrating intense FDG uptake and other malignant tumors such as adenoid cystic carcinoma showing little FDG avidity.
Paraganglioma
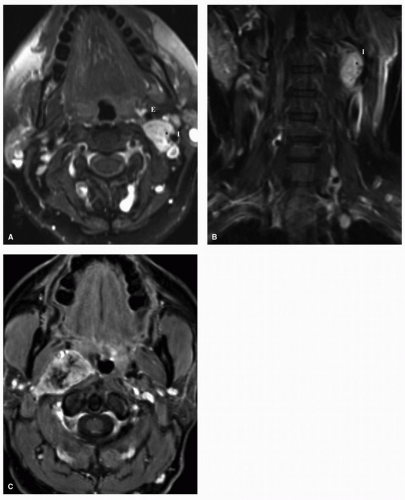
Paragangliomas, also known as glomus tumors, are tumors of neural crest origin that have a chemoreceptor function and most commonly arise at the carotid body bifurcation (glomus caroticum), the jugular foramen (glomus jugulare), along the course of the vagus nerve (glomus vagale), and within the middle ear space (glomus tympanicum). In contrast to paragangliomas arising from the lower mediastinum, abdomen, or pelvis, the vast majority of head and neck paragangliomas do not secrete catecholamines. Paragangliomas can rarely occur in association with genetic syndromes such as von Hippel-Lindau syndrome, neurofibromatosis type I, MEN2A, and MEN2B. Nonsyndromic paragangliomas occur more frequently and may be caused by genetic mutations involving the hereditary paraganglioma (PGL) genes that code for the succinate dehydrogenase (SDH) enzyme. Genes that have been identified include SDHD gene 1 (PGL1) on chromosome 11q23, SDHB gene (PGL4) on chromosome 1p35-p36, SDHC gene (PGL3) on chromosome 1q21, and the PGL2 gene, of which the exact location on chromosome 11 remains unknown. PGL3 and PGL4 are inherited in an autosomal dominant form, whereas PGL1 and PGL2 are subject to maternal imprinting. As such, children inheriting the PGL1 and PGL2 genes only express the phenotype if the diseased gene is inherited from the father.17 SDHD and SDHB mutations show an age-related penetrance showing 86% and 77% penetrance by age 50, respectively.18 SDHD mutation carriers manifest head and neck paragangliomas and multifocal disease more commonly (Fig. 22.6), whereas SDHB carriers have an increased frequency of malignant paragangliomas in addition to a predisposition toward thyroid cancer and renal cancer.18,19
Screening for SDH mutations should be carried out in family members of patients with a clinical history of familial paraganglioma, those with an age of presentation below 45 years, and those with multiple synchronous or metachronous tumors. SDHB mutation testing should be considered in
all patients with a malignant tumor. In individuals known to have familial paraganglioma, genetic testing should commence at age 10 or at least 10 years before the earliest age of diagnosis in relatives affected. Periodic clinical, biochemical, and imaging surveillance has been recommended to include 24-hour urinary excretion of fractionated metanephrines and catecholamines and MRI or CT scan imaging of the skull base every 2 years and MRI or CT imaging of the thorax, abdomen, and pelvis every 4 years.20 Meta-iodinated benzylguanidine (MIBG) scintigraphy has traditionally been used to detect paragangliomas or metastatic disease not visualized on CT scan or MRI with sensitivities between 77% and 90% and specificity between
88% and 96%, with the addition of SPECT improving sensitivities to between 88% and 96%.21 Over the past decade, there has been an increasing interest in the use of positron emission tomography (PET) as an alternative to MIBG scintigraphy, with PET imaging shown to have a higher sensitivity in detecting distant metastasis in patients with SDHB mutations.22,23
all patients with a malignant tumor. In individuals known to have familial paraganglioma, genetic testing should commence at age 10 or at least 10 years before the earliest age of diagnosis in relatives affected. Periodic clinical, biochemical, and imaging surveillance has been recommended to include 24-hour urinary excretion of fractionated metanephrines and catecholamines and MRI or CT scan imaging of the skull base every 2 years and MRI or CT imaging of the thorax, abdomen, and pelvis every 4 years.20 Meta-iodinated benzylguanidine (MIBG) scintigraphy has traditionally been used to detect paragangliomas or metastatic disease not visualized on CT scan or MRI with sensitivities between 77% and 90% and specificity between
88% and 96%, with the addition of SPECT improving sensitivities to between 88% and 96%.21 Over the past decade, there has been an increasing interest in the use of positron emission tomography (PET) as an alternative to MIBG scintigraphy, with PET imaging shown to have a higher sensitivity in detecting distant metastasis in patients with SDHB mutations.22,23
Histologically, paragangliomas consist of two different cell types organized into clusters separated by a fibrovascular stroma pathognomonically known as Zellballen (“cell balls” in German). Type I cells, also known as chief cells, have a polygonal shape and eosinophilic cytoplasm and are concentrated in the center. Type II, or sustentacular cells, are spindle
shaped and surround the periphery of the Zellballen. Rarely, paragangliomas can secrete catecholamines and may present with headaches, palpitation, tremor, and profuse sweating. Patients with these symptoms should be tested for 24-hour urinary collection of metanephrine, normetanephrine, and vanillylmandelic acid (VMA) and plasma free metanephrines.
shaped and surround the periphery of the Zellballen. Rarely, paragangliomas can secrete catecholamines and may present with headaches, palpitation, tremor, and profuse sweating. Patients with these symptoms should be tested for 24-hour urinary collection of metanephrine, normetanephrine, and vanillylmandelic acid (VMA) and plasma free metanephrines.
These tumors have a lobulated, ovoid appearance on MRI with well-defined margins. On T1 sequences they have signal intensity similar to that of muscle, whereas on T2 sequences they are mildly hyperintense. They enhance rapidly and intensely with gadolinium contrast and larger tumors possess punctuate areas of flow voids, which may confer the classic “salt-and-pepper”
appearance (Figs. 22.7 and 22.8). Surgical biopsy of these lesions should be avoided, as they are highly vascular. A mass splaying the internal and external carotid arteries at the bifurcation (i.e., lyre sign) is highly suggestive of a carotid body tumor.
appearance (Figs. 22.7 and 22.8). Surgical biopsy of these lesions should be avoided, as they are highly vascular. A mass splaying the internal and external carotid arteries at the bifurcation (i.e., lyre sign) is highly suggestive of a carotid body tumor.
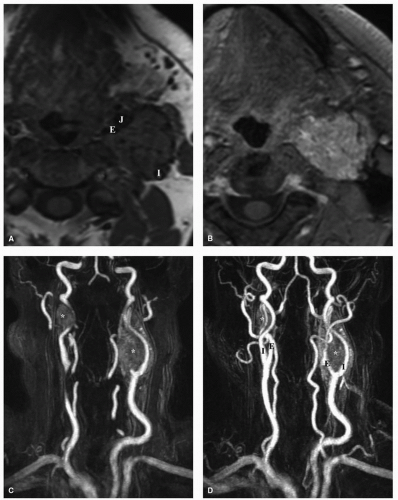
Shamblin’s classification of carotid body tumors based on their relationship to the internal carotid artery (ICA) has recently been correlated with MR imaging. In type I tumors, the area of contact with the ICA is ≤180 degrees, type II tumors contact the ICA 180 to 270 degrees, and type III tumors have an area of contact >270 degrees.24 Larger type II or type III tumors have been shown to have an increased rate of intraoperative ICA sacrifice, and preoperative balloon occlusion testing
is therefore recommended to assess the integrity of collateral circulation to the brain and plan for possible carotid artery replacement preoperatively. In retrospective reviews of patients who experienced intraoperative ICA disruption without undergoing balloon occlusion testing, it is shown that 26% suffered a stroke with a subsequent mortality rate of 12%, compared to a 4.7% stroke rate alone with no mortalities for patients who passed a total balloon occlusion test.25,26 A wide variety of methods incorporating CT imaging and MRI of cerebral blood flow during balloon occlusion testing and interpretation criteria have been used to assess the adequacy of cerebral blood flow during balloon occlusion testing. No one technique has been demonstrated as being superior in predicting the incidence of permanent neurologic deficit following ICA sacrifice.
is therefore recommended to assess the integrity of collateral circulation to the brain and plan for possible carotid artery replacement preoperatively. In retrospective reviews of patients who experienced intraoperative ICA disruption without undergoing balloon occlusion testing, it is shown that 26% suffered a stroke with a subsequent mortality rate of 12%, compared to a 4.7% stroke rate alone with no mortalities for patients who passed a total balloon occlusion test.25,26 A wide variety of methods incorporating CT imaging and MRI of cerebral blood flow during balloon occlusion testing and interpretation criteria have been used to assess the adequacy of cerebral blood flow during balloon occlusion testing. No one technique has been demonstrated as being superior in predicting the incidence of permanent neurologic deficit following ICA sacrifice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree