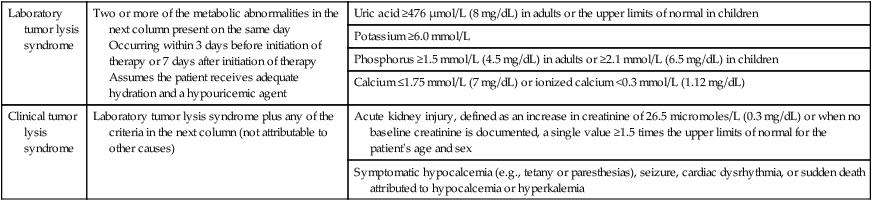
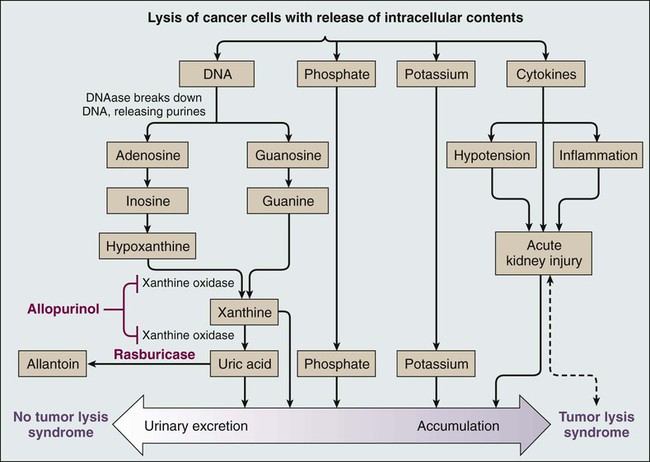
• Tumor lysis syndrome can occur in any patient with newly diagnosed or relapsed cancer, and thus all patients should undergo risk stratification and management according to their risk for clinical tumor lysis syndrome. • Laboratory tumor lysis syndrome is defined as the presence of two or more of the following abnormalities present on the same day: hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia as a result of hyperphosphatemia. • Clinical tumor lysis syndrome is defined as laboratory tumor lysis syndrome plus acute kidney injury, symptomatic hyperkalemia, or symptomatic hypocalcemia, and it should be prevented whenever possible. • The incidence of clinical tumor lysis syndrome depends on the number of risk factors present at presentation and on the management of patients potentially at risk. • Risk factors for clinical tumor lysis syndrome include a large cancer mass, high cell lysis potential (chemosensitivity), and patient factors (e.g., preexisting nephropathy, dehydration, acidosis, hypotension, and nephrotoxin exposure). • Management depends on the risk of the development of clinical tumor lysis syndrome: Tumor lysis syndrome is a potentially fatal metabolic condition that occurs in patients with rapidly proliferating, bulky, or chemosensitive tumors (Table 38-1).1 It has been most commonly reported in patients with high-grade non-Hodgkin lymphomas (NHL) and acute leukemias, but it can occur in persons with virtually any type of cancer when a large cancer cell mass is present and the cancer is sensitive to initial therapy. Table 38-1 Modified Cairo-Bishop Classification of Tumor Lysis Syndrome Data from reference 1. Tumor lysis syndrome occurs most commonly after treatment with cytotoxic therapy, but it can also occur spontaneously in patients with highly proliferative tumors. By releasing tumor cellular components into the bloodstream, tumor lysis syndrome results in metabolic abnormalities including hyperphosphatemia, hyperkalemia, hypocalcemia, hyperuricemia, and azotemia (Fig. 38-1). Acute kidney injury, seizures, cardiac arrhythmias, nausea, and vomiting may occur as a result of these metabolic abnormalities. To reduce morbidity and mortality, early diagnosis and identification of patients at risk for tumor lysis syndrome are of the utmost importance.1,2 The catabolism of nucleic acids ultimately results in the production of uric acid.3 Purines are first degraded into hypoxanthine, then xanthine, and finally into uric acid through the action of xanthine oxidase.4,5 Hyperuricemia leads to the deposition of uric acid crystals in the renal tubules because of the poor solubility of uric acid and can result in acute kidney injury.4 Hyperphosphatemia can also cause acute kidney injury. Phosphates combine with calcium, generating calcium phosphate salts that deposit in the renal tubules. The binding of calcium by phosphate leads to hypocalcemia, which in turn can cause vomiting, muscle cramps, tetany, paresthesias, seizures, and cardiac dysrhythmias.6 Hyperkalemia from cellular lysis can lead to cardiac dysrhythmias, ventricular tachycardia, fibrillations, or cardiac arrest.6 Several risk factors for tumor lysis syndrome have been identified, including tumor-related factors, individual patient characteristics, and the type of chemotherapy used.7 Certain tumor types have historically been associated with an increased risk for the development of tumor lysis syndrome, including Burkitt leukemia, acute lymphoblastic leukemia (ALL), acute myeloid leukemia (especially those with inv(16) chromosomal translocation), and NHL (particularly Burkitt lymphoma)10–10; patients with these tumor types were the most frequently enrolled in compassionate-use trials of hypouricemic agents.11,12 However, tumor lysis syndrome can also develop in patients with chronic lymphocytic leukemia (CLL) and chronic myelogenous leukemia.13 Acute leukemias and high-grade lymphomas often have a high proliferative rate, a large tumor burden, and are particularly sensitive to chemotherapy; the combination of high tumor bulk and sensitivity to therapy make tumor lysis syndrome more likely.14 The high nucleic acid, phosphorus content, and increased activity of purine metabolism in these tumors predispose patients to tumor lysis syndrome.7 In a study of 102 patients with high-grade NHL who received prophylactic hyperhydration and allopurinol, the incidence of clinically significant tumor lysis syndrome was 6%, and one patient required dialysis.15 A retrospective study of 755 patients with NHL or acute leukemia who received rasburicase for prevention or treatment of tumor lysis syndrome documented a 5.3% incidence of tumor lysis syndrome (including both laboratory and clinical tumor lysis syndrome).16 Seven patients in this study died from tumor lysis syndrome (0.9% of all patients and 17.5% of patients with tumor lysis syndrome).16,17 In a multinational study of 235 pediatric patients with advanced-stage, B-cell NHL who were treated with the same chemotherapy regimen, 27% and 15% of U.S. patients (who all received allopurinol) experienced tumor lysis syndrome and required dialysis, respectively, whereas 11% and 3% of French patients (who all received rasburicase) experienced tumor lysis syndrome and required dialysis, respectively.18 Hence the risk for tumor lysis syndrome depends not only on tumor-related risk factors but also on the supportive care used (Fig. 38-1). In addition to tumor-related risk factors for tumor lysis syndrome, patient-related factors affect tumor lysis syndrome risk, including leukocytosis, hyperuricemia, elevated serum lactate dehydrogenase, elevated serum creatinine, renal insufficiency, dehydration, acidic urine, and decreased urinary flow at the time of presentation.7,14 Finally, specific cytotoxic agents have been associated with tumor lysis syndrome in particular diseases, such as fludarabine and lenalidomide in patients treated for CLL.7,13,19,20 Because the risk of clinical tumor lysis syndrome is proportional to the rapidity of response to therapy, patients whose tumors respond quickly to a new therapeutic agent have an increased risk for the development of tumor lysis syndrome. Patients with diagnoses not typically associated with tumor lysis syndrome (e.g., metastatic colon cancer) can also be affected by tumor lysis syndrome when the treatment includes new, highly active agents, such as cetuximab.21 To successfully treat and prevent tumor lysis syndrome, it is necessary to identify patients at risk and tailor prevention according to risk, as described in the next section.6 Published guidelines for the diagnosis, prevention, and management of tumor lysis syndrome (Table 38-2) differ in many details. However, all guidelines agree that patients at risk for tumor lysis syndrome should undergo risk stratification and management on the basis of their risk. Clinical tumor lysis syndrome is the outcome that should be prevented, because by definition it is associated with morbidity. Standard prophylaxis includes close monitoring, hyperhydration to increase urine output and facilitate renal excretion of uric acid and phosphorus, and administration of a hypouricemic agent, such as allopurinol or rasburicase, to prevent the formation of uric acid crystals in the kidney, as well as administration of phosphate binders to reduce calcium phosphate precipitation in the kidneys.1,2,4,22 Both allopurinol and rasburicase have been used successfully to reduce the incidence of tumor lysis syndrome in pediatric and adult patients.1,23 Historically, urine has been alkalinized by the administration of bicarbonate to improve the solubility of uric acid (Fig. 38-2)21,24
Tumor Lysis Syndrome
 Negligible risk—no prophylaxis, no monitoring
Negligible risk—no prophylaxis, no monitoring
 Low risk (1% risk for clinical tumor lysis syndrome)—hyperhydration, allopurinol, and daily laboratory evaluation
Low risk (1% risk for clinical tumor lysis syndrome)—hyperhydration, allopurinol, and daily laboratory evaluation
 Intermediate risk—hyperhydration, rasburicase, inpatient monitoring, and laboratory evaluation every 8 to 12 hours
Intermediate risk—hyperhydration, rasburicase, inpatient monitoring, and laboratory evaluation every 8 to 12 hours
 High risk—hyperhydration, rasburicase, cardiac monitoring on the inpatient ward, laboratory evaluation every 6 to 8 hours, and rapid access to hemodialysis
High risk—hyperhydration, rasburicase, cardiac monitoring on the inpatient ward, laboratory evaluation every 6 to 8 hours, and rapid access to hemodialysis
 Established clinical tumor lysis syndrome at presentation—hyperhydration, rasburicase, cardiac monitoring in the intensive care unit, laboratory evaluation every 4 to 6 hours, and rapid access to hemodialysis
Established clinical tumor lysis syndrome at presentation—hyperhydration, rasburicase, cardiac monitoring in the intensive care unit, laboratory evaluation every 4 to 6 hours, and rapid access to hemodialysis
Epidemiology and Definition
Laboratory tumor lysis syndrome
Two or more of the metabolic abnormalities in the next column present on the same day
Occurring within 3 days before initiation of therapy or 7 days after initiation of therapy
Assumes the patient receives adequate hydration and a hypouricemic agent
Uric acid ≥476 µmol/L (8 mg/dL) in adults or the upper limits of normal in children
Potassium ≥6.0 mmol/L
Phosphorus ≥1.5 mmol/L (4.5 mg/dL) in adults or ≥2.1 mmol/L (6.5 mg/dL) in children
Calcium ≤1.75 mmol/L (7 mg/dL) or ionized calcium <0.3 mmol/L (1.12 mg/dL)
Clinical tumor lysis syndrome
Laboratory tumor lysis syndrome plus any of the criteria in the next column (not attributable to other causes)
Acute kidney injury, defined as an increase in creatinine of 26.5 micromoles/L (0.3 mg/dL) or when no baseline creatinine is documented, a single value ≥1.5 times the upper limits of normal for the patient’s age and sex
Symptomatic hypocalcemia (e.g., tetany or paresthesias), seizure, cardiac dysrhythmia, or sudden death attributed to hypocalcemia or hyperkalemia
Etiology and Pathogenesis
Risk Factors and Incidence of Tumor Lysis Syndrome
Prevention and Management of Tumor Lysis Syndrome
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine