Highlights
- •
Tumor location at TURBT predicts ipsilateral lymph node metastases.
- •
Right-sided tumors show more positive ipsilateral lymph nodes.
- •
Left-sided tumors also predict increased ipsilateral node positivity.
- •
This is a hypothesis-generating study.
- •
Findings would suggest lateral tumors need focused lymph node evaluation.
Abstract
Objective
To assess whether tumor location at diagnostic TURBT is predictive of ipsilateral nodal involvement in patients who underwent radical cystectomy (RC) with lymph-nodes dissection for bladder cancer (BCa).
Materials and methods
All patients who underwent RC for BCa at a single institution between 2014–2023 were assessed. Tumor location at TURBT was defined as right-sided, median-line, left-sided, and diffused. Distribution in the percentage of ipsilateral positive lymph-nodes and number of ipsilateral positive lymph-nodes between tumor locations were assessed with Kruskal-Wallis tests. Linear regressions were fitted to assess whether left or right location, compared to the remaining locations grouped, was associated to the percentage and number of positive ipsilateral lymph-nodes.
Results
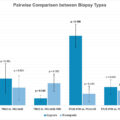
239 patients were included. The number of ipsilateral positive lymph nodes was superior in right-sided tumors when compared to the rest of the bladder (0, I.Q.R. 0–1 vs. 0, I.Q.R. 0–0, P = 0.047), as well as the percentage of ipsilateral positive lymph-nodes (0, I.Q.R. 0–14.3 vs. 0, I.Q.R. 0–3.7, P = 0.042). The number of ipsilateral positive lymph-nodes in left-sided tumors was superior when compared to the rest of the bladder (0, I.Q.R. 0–1 vs. 0, I.Q.R. 0–0, P = 0.02), as well as the percentage (0, I.Q.R. 0–13.7 vs. 0, I.Q.R. 0–0, P = 0.036). At linear regression analyses, right- and left-sided tumors were associated with an increased percentage of ipsilateral positive lymph-nodes ( P = 0,019 and P = 0,003) out of the total ipsilateral lymph-nodes excised.
Conclusions
Lateral wall tumor location at diagnostic TURBT (either right or left side) predicts a higher percentage of ipsilateral positive lymph-nodes s/p RC.
1
Introduction
Radical Cystectomy (RC) with pelvic lymph-Nodes dissection (PLND) and urinary diversion is the treatment of choice in T2–T4a, N0M0 muscle-invasive bladder cancer, and in very high-risk nonmuscle invasive bladder cancer (NMIBC), BCG-refractory, BCG-relapsing, and BCG-unresponsive NMIBC [ , ].
Currently, the standard template of PLND during RC includes the excision of lymph-nodes cranially up to the common iliac bifurcation, with the ureter being the medial border, and including the internal iliac, presacral, obturator fossa, and external iliac nodes [ ].
Limited, extended, and super-extended PLND have been proposed as alternatives in selected cases; the first includes the nodes from the true pelvis but does not comprise the deep obturator lymph-nodes. The second includes all lymph-nodes as the standard one, but its cranial limit is the aortic bifurcation, while the third reaches the inferior mesenteric artery [ , ].
The primary tumor’s access to lymphatic and vascular tissue has been suggested as a major contributing factor to the disease spread in loco-regional lymph-nodes: data supporting the correlation between the presence of lymphovascular invasion within the primary tumor and pelvic lymph-nodal involvement is present [ , ].
Moreover, while the diagnostic value of PLND is well established, its therapeutic effect is still debated. While evidence suggests better oncological outcomes in patients treated with PLND, there’s no clear evidence on the impact of different PLND templates [ ].
However, scant and heterogeneous data are available on whether tumor endoluminal location is both prognostically valuable and predictive of lymph-nodal spread [ ]; indeed, no efforts have been reported on unilaterally modulating the extension of PLND templates based on the tumor’s endoluminal location in select cases.
Understanding the relationship between tumor location at the time of its transurethral resection and the respective laterality of lymph-nodal metastases could aid clinicians in risk stratification and surgical planning. Our study aimed to shed some insight into this matter.
2
Methods
2.1
Patient cohort
From our prospectively-maintained institutional bladder cancer database, we extracted and retrospectively analyzed all patients who underwent RC with bilateral standard PLND – as per institutional protocol – between 2014 and 2023.
Only patients with available data regarding tumor location at TURBT, and lymph-nodal status at final pathology report s/p RC were included in the analysis.
Patients who were clinically node-positive and/or who had metastatic disease at preoperative staging were excluded. Also, patients who underwent neoadjuvant chemotherapy were excluded from the analysis.
Specifically for the purpose of the study, tumor endoluminal location at the last TURBT performed was defined as:
- –
right-sided, which included disease involving the right bladder wall and/or the right trigone;
- –
median-line, including disease of the central trigone, the posterior wall, the dome and/or the anterior wall;
- –
left-sided, with disease involving the left bladder wall and/or the left trigone;
- –
diffuse, multi-focal disease.
2.2
Lymph node dissection
Lymph node dissection was carried out according to the standard template cited in the introduction. While in some cases, pelvic lymph nodes were excised en-bloc (n=43), in the majority of the patients (n=196), they were individually grouped following a standardized anatomical template, comprising external iliac, internal iliac plus obturator, presacral, Marcille’s common iliac, and Cloquet’s nodal stations bilaterally. Specimens were immersed in formalin, enumerated, and then examined for cancer infiltration, with staging assigned in accordance with the 2017 version of the TNM system (8th edition).
2.3
Outcome
The primary outcome was to assess whether tumor endoluminal location at TURBT was predictive of ipsilateral pelvic lymph-nodal involvement.
2.4
Covariates
Relevant demographic and tumor-related data were considered to account for the potential effect of confounders. Demographic data included patient age (at the time of surgery), gender, ASA score, and Charlson’s comorbidity index. Tumor-related characteristics included endoscopic tumor size (in mm), pT stage, 2016 WHO Grading, histological subtype at TURBT and RC, and number of nodes excised during PLND. The year of surgery was also collected.
2.5
Statistical analysis
Descriptive statistics were used to characterize the study cohort, stratified by tumor location; frequencies and proportions were used for categorical data, while medians, interquartile ranges (IQR), and 5°–95° centiles were used for continuous data. Kruskal-Wallis and chi-square tests were used to compare the statistical significance of differences in the distribution of continuous and categorical variables, respectively, between different endoluminal tumor locations. Distribution in the percentage of ipsilateral positive lymph-nodes and in the number of ipsilateral positive lymph-nodes between different tumor locations was assessed with Kruskal-Wallis. Linear regressions were fitted to assess whether the left or right location, compared to the rest of the locations grouped, was associated with an increased number of ipsilateral positive lymph-nodes and an increased percentage of ipsilateral positive lymph-nodes out of the total number of nodes excised; Beta coefficients and 95%C.I. have been reported.
All statistical tests were performed using SPSS IBM SPSS Statistics version 23.0 (Armonk, NY: IBM Corp.). All tests were two-sided with a significance level set at P < 0.05.
3
Results
From a total of 314 patients in the institutional database, 239 patients were included in the analysis after accounting for inclusion/exclusion criteria.
Of these, 64 patients (27%) had a right-sided tumor at TURBT, 84 (35.4%) had a median-line tumor, 69 (29.1%) had a left-side tumor, and 20 (8.4%) had diffuse disease. After TURBT, it was found that 206 (86%) patients were affected by urothelial carcinoma, 12 (5.1%) by urothelial carcinoma with partial squamous differentiation, and 4 (1,7%) by sarcomatoid urothelial carcinoma, with no statistically significant difference between tumor location ( Table 1 ).
| Variable | Overall | Right side | Central | Left side | Diffuse | P value |
|---|---|---|---|---|---|---|
| Age | 0.052 | |||||
| Median | 71 | 69.5 | 71 | 75 | 72 | |
| IQR | 63–77 | 64–74.7 | 63–77 | 68–80 | 53–76 | |
| Centiles (5°–95°) | 50–85 | 49–83 | 52.2–84.7 | 49–85.4 | 49.1–88.5 | |
| Gender | 0.9 | |||||
| Male | 191 (79.9) | 53 (82.8) | 67 (79.8) | 55 (77.5) | 16 (80) | |
| Female | 48 (20.1) | 11 (20.2) | 17 (22.5) | 16 (22.5) | 4 (20) | |
| ASA score | 0.71 | |||||
| 1 | 4 (1.7) | 2 (3.1) | 1 (1.2) | 0 (0) | 1 (5) | |
| 2 | 135 (56.5) | 37 (57.8) | 51 (60.7) | 39 (54.9) | 8 (40) | |
| 3 | 88 (36.8) | 22 (34.4) | 28 (33.3) | 28 (39.4) | 10 (50) | |
| 4 | 12 (5) | 3 (4.7) | 4 (4.8) | 4 (5.6) | 1 (5) | |
| CCI | 0.35 | |||||
| Median | 6 | 6 | 5 | 6 | 6 | |
| IQR | 5–7 | 4–7 | 5–6 | 5–7 | 4–7 | |
| Centiles (5°–95°) | 3–9.7 | 2.6–11.35 | 3–9.5 | 3.8–9.1 | 3–8.5 | |
| Endoscopic tumour size (mm) | 0.48 | |||||
| Median | 30 | 30 | 30 | 32.5 | 15 | |
| IQR | 15–45 | 17.5–47.5 | 16.2–40 | 42.5 | 10–80 | |
| Centiles (5°–95°) | 10–67.5 | 10–60 | 10–70 | 8.9–61 | 10–92 | |
| pT at TURBT | 0.054 | |||||
| pTa | 21 (8.8) | 5 (7.8) | 8 (9.6) | 3 (4.2) | 5 (25) | |
| CIS only | 7 (2.9) | 1 (1.6) | 1 (1.2) | 3 (4.2) | 2 (10) | |
| pT1 | 64 (26.9) | 13 (20.3) | 23 (27.7) | 18 (25.4) | 10 (50) | |
| pT2a | 46 (19.3) | 14 (21.9) | 17 (205) | 13 (18.3) | 2 (10) | |
| pT2b | 94 (39.5) | 29 (45.3) | 32 (38.6) | 32 (45.1) | 1 (5) | |
| pT3a | 4 (1.7) | 1 (1.6) | 1 (1.2) | 2 (2.8) | 0 (0) | |
| pT3b | 2 (0.8) | 1 (1.6) | 1 (1.2) | 0 (0) | 0 (0) | |
| pT4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Grading (WHO) at TURBT | 0.85 | |||||
| LG | 11 (4.8) | 2 (3.2) | 5 (6.3) | 3 (4.4) | 1 (5) | |
| HG | 219 (95.2) | 61 (96.8) | 74 (93.7) | 65 (95.6) | 19 (95) | |
| histological subtype both at TURBT | 0.51 | |||||
| urothelial Ca | 209 (87.4) | 53 (82.8) | 74 (88.1) | 62 (87.3) | 20 (100) | |
| urothelial Ca with partial squamous differentiation | 13 (5.4) | 7 (10.9) | 2 (2.4) | 4 (5.6) | 0 (0) | |
| sarcomatoid urothelial Ca | 3 (1.3) | 0 (0) | 1 (1.2) | 2 (2.8) | 0 (0) | |
| Other | 14 (5,9) | 4 (6.3) | 7 (8.3) | 3 (4.2) | 0 (0) | |
| pT at RC | 0.2 | |||||
| pT0 | 26 (10,9) | 4 (6.3) | 10 (11.9) | 8 (11.3) | 4 (20) | |
| pTa | 2 (0.8) | 0 (0) | 1 (1.2) | 0 (0) | 1 (5) | |
| CIS only | 15 (6.3) | 1 (1.6) | 9 (10.7) | 4 (5.6) | 1 (5) | |
| pT1 | 33 (13.8) | 8 (12.5) | 10 (11.9) | 11 (15.5) | 4 (20) | |
| pT2a | 18 (7.5) | 3. (4.7) | 10 (11.9) | 3 (4.2) | 2 (10) | |
| pT2b | 15 (6.3) | 3 (4.7) | 7 (8.3) | 3 (4.2) | 2 (10) | |
| pT3a | 26 (10.9) | 8 (12.5) | 5 (6) | 12 (16.9) | 1 (5) | |
| pT3b | 47 (19.7) | 20 (31.3) | 13 (15.5) | 13 (18.3) | 1 (5) | |
| pT4a | 42 (17.6) | 12 (18.8) | 13 (15.5) | 14 (19.7) | 3 (15) | |
| pT4b | 1 (0.4) | 0 (0) | 1 (1.2) | 0 (0) | 0 (0) | |
| Grading (WHO) at RC | 0.9 | |||||
| LG | 8 (3.77) | 2 (3.0) | 2 (2.4) | 3 (4.1) | 1 (5.6) | |
| HG | 182 (85.8) | 49 (74.3) | 63 (75.9) | 56 (77.8) | 14 (77.8) | |
| missing | 57 (10.4) | 13 (23.7) | 19 (22.9) | 12 (16.7) | 5 (16.7) | |
| pN | 0.68 | |||||
| pN0 | 162 (67.8) | 40 (62.5) | 60 (71.4) | 45 (63.4) | 17 (85) | |
| pN1 | 23 (9.6) | 8 (12.5) | 8 (9.5) | 6 (8.45) | 1 (5) | |
| pN2 | 37 (15.5) | 12 (18.8) | 10 (11.9) | 14 (19.7) | 1 (5) | |
| pN3 | 17 (7.1) | 4 (6.2) | 6 (7.1) | 6 (8.45) | 1 (5) | |
| Histological subtype at RC | 0.69 | |||||
| Urothelial Ca | 139 (58.2) | 34 (53.1) | 52 (61.9) | 41 (57.7) | 12 (60) | |
| Urothelial Ca with partial squamous differentiation | 34 (14,2) | 16 (25) | 8 (9.5) | 9 (12.7) | 1 (5) | |
| No residual disease | 26 (10,9) | 4 (6.3) | 10 (11.9) | 8 (11.3) | 4 (20) | |
| Other | 40 (16.7) | 10 (15.6) | 14 (16.6) | 13 (18.3) | 3 (15) | |
| Number of PLN excised at RC | 0.73 | |||||
| Median | 23 | 22 | 22.5 | 23 | 27 | |
| IQR | 16–31 | 12–31 | 17–29.25 | 16–28 | 14–37 | |
| Centiles (5°–95°) | 6.2–47.6 | 5–48 | 7.8–46.6 | 8.4–48.6 | 2–42 | |
| Year of surgery | 0.92 | |||||
| Median | 2017 | 2017 | 2017 | 2017 | 2017 | |
| IQR | 2016–2019 | 2016–2019 | 2016–2019 | 2016–2018 | 2016–2018 | |
| Centiles (5°–95°) | 2014–2022 | 2015–2022 | 2015–2022 | 2014–2022 | 2015–2023 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree