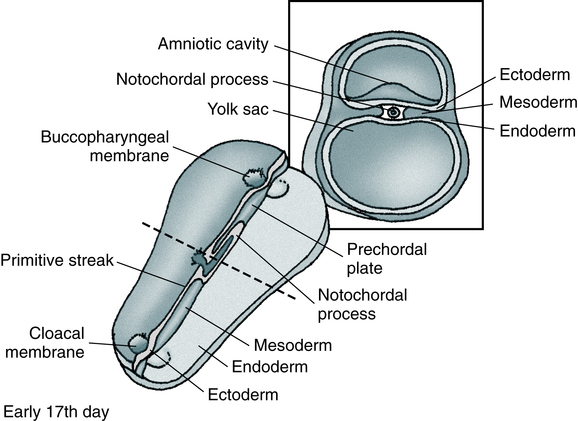
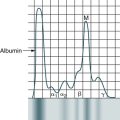
At the conclusion of this chapter, the reader should be able to: • Compare the characteristics of benign and malignant tumors. • Describe the epidemiology of cancer in adults and children. • Explain the characteristics of the three major causative factors in human cancer. • Compare the stages of carcinogenesis. • Describe the aspects of cancer-related genes. • Define and give examples of proto-oncogenes. • Describe the role of oncogenes. • Describe the characteristics of the major body defenses against cancer. • Identify and discuss the characteristics of tumor markers. • Discuss what’s new in cancer diagnostic testing. • Compare various modalities for treating cancer. • Analyze representative case studies. • Correctly answer case study related multiple choice questions. • Be prepared to participate in a discussion of critical thinking questions. • Describe the principle and clinical applications of the prostate-specific antigen procedure. Oncology is that branch of medicine devoted to the study and treatment of tumors. The term tumor is commonly used to describe a proliferation of cells that produces a mass rather than a reaction or inflammatory condition. Tumors are neoplasms and are described as benign or malignant. Most tumors are of epithelial origin (ectoderm, endoderm, or mesoderm); the remaining tumors are of connective tissue origin (Fig. 33-1). The key distinction between benign and malignant tumors is the ability of malignant tumors to invade normal tissue and metastasize to other secondary sites. • Self-renewal when daughter cells retain the same biologic properties as the parent cell • Capability to develop into multiple lineages If normal self-renewal is subverted, it becomes abnormal self-renewal. If increased self-renewal occurs, combined with the intrinsic growth potential of stem cells, it may yield a malignant phenotype. It is possible that cancer stem cells can arise by mutation from normal stem cells or mutated progenitor cells (Fig. 33-2). Benign tumors are characterized by the following: Malignant tumors are characterized by the following: • Increase in the number of cells that accumulate • Usually, invasion of tissues • Dissemination by lymphatic spread or by seeding within a body cavity • Characteristic nuclear cellular features • Receptors for integrin molecules (e.g., fibronectin), which help malignant cells adhere to extracellular matrix, type IV collagenases, which dissolve basement membranes, and proteases • Secretion of transforming growth factor α (TGF-α) and transforming growth factor β (TGF-β) to promote angiogenesis and collagen deposition • Often, recurrence after attempts to eradicate the tumor by surgery, radiation, or chemotherapy Biologically distinct and relatively rare populations of tumor-initiating cells have been identified in cancers of the hematopoietic system, brain, and breast. Cells of this type have the capacity for self-renewal, the potential to develop into any cell in the overall tumor population, and the proliferative ability to drive continued expansion of the population of malignant cells. The properties of these tumor-initiating cells closely parallel the three features that define normal stem cells. Malignant cells with these functional properties are termed cancer stem cells (Fig. 33-3). Cancer stem cells can be the source of all the malignant cells in a primary tumor. The incidence of cancer has been correlated with certain environmental factors. Table 33-1 lists environmental factors that have been definitively linked with cancer, including aerosol and industrial pollutants, drugs, and infectious agents. Radiation exposure is also known to be associated with specific types of cancer (e.g., acute leukemia, thyroid cancer, sarcomas, breast cancer). Women concerned about organochlorine substances (e.g., polychlorinated biphenyls [PCBs], dioxins, pesticides [DDT, banned in 1972]) can be reassured that available evidence does not suggest an association between exposure to these chemicals and breast cancer. Table 33-1 Selected Environmental Factors Associated With Cancer The incidence of cancer is 10,000 times greater than expected in patients with an immunodeficiency syndrome. The increased incidence of lymphomas in congenital, acquired, and drug-induced immunosuppression is consistent with the failure of normal immune mechanisms or antigen overstimulation with a loss of normal feedback control. Table 33-2 lists other cancer-related conditions. Table 33-2 Viral causes of some cancers are known. Viruses associated with specific cancers are listed in Table 33-1. Nonpermissive cells that prevent an oncogenic RNA or DNA virus from completing its replication cycle often produce changes in the genome that result in the activation of proto-oncogenes or inactivation of suppressor genes. Cancer-predisposing genes may act in the following ways: • Affect the rate at which exogenous precarcinogens are metabolized to actively carcinogenic forms that can damage the cellular genome directly • Affect a host’s ability to repair resulting damage to DNA • Alter the immune ability of the body to recognize and eradicate incipient tumors • Affect the function of the apparatus responsible for the regulation of normal cell growth and associated proliferation of tissue Relatively few cancer-predisposing genes have been described. An absence of functional alleles at specific loci, however, allows the genesis of the malignant process (Table 33-3). For example, individuals with certain mutations in the gene BRCA2 are at a very high risk (up to 85%) for developing breast cancer and other cancers (e.g., ovarian cancer) because a DNA repair path cannot properly repair ongoing wear and tear to the DNA. Table 33-3 Tumors Associated With Homozygous Loss of Specific Chromosomal Loci Once an oncogene is activated by mutation, it promotes excessive or inappropriate cell proliferation. Oncogenes have been detected in about 15% to 20% of a variety of human tumors and appear to be responsible for specifying many of the malignant traits of these cells. More than 30 distinct oncogenes, some of which are associated with specific tumor types, have been identified (Table 33-4). Each gene has the ability to evoke many of the phenotypes characteristic of cancer cells. Table 33-4 Some Oncogenes Formed by Somatic Mutation of Normal Genetic Loci • Growth factors (e.g., sis oncogene) • Epidermal growth factor receptors (EGFRs) • Membrane-associated protein kinases (e.g., src oncogene) • Membrane-related guanine triphosphate (GTP)–binding proteins (e.g., ras oncogene) • Cytoplasmic protein kinases (e.g., ras oncogene) • Transcription regulators located in the nucleus (e.g., c-myc oncogene) • Overexpression of the c-erbB-2 (HER2/neu) oncogene is noted in up to 34% of patients with invasive ductal breast carcinoma and predicts poor survival. • Activation of the ras proto-oncogene (point mutation) is associated with about 30% of all human cancers. About 25% of patients with acute myelogenous leukemia display this point mutation. Ras is mutated frequently in colon and pancreatic cancers; it appears that ras activation leads to unregulated expression of IL-24 and its receptors. • Translocation of the abl proto-oncogene from chromosome 9 to chromosome 22 with formation of a large bcr-abl hybrid gene on chromosome 22 (Philadelphia chromosome) results in chronic myelogenous leukemia. • Inactivation of suppressor genes (point mutations) leads to unrestricted cell division, inactivation of each of the RB1 suppressor genes on chromosome 13 is associated with malignant retinoblastoma in children, and inactivation of the p53 suppressor gene on chromosome 17 accounts for 25% to 50% of all malignancies involving the colon, breast, lung, and central nervous system. Although there is no single satisfactory explanation for the success of tumors in escaping the immune rejection process, it is believed that early clones of neoplastic cells are eliminated by the immune response. The growth of malignant tumors is primarily determined by the proliferative capacity of the tumor cells and by the ability of these cells to invade host tissues and metastasize to distant sites. It is believed that malignant tumors can evade or overcome the mechanisms of host defenses (Color Plate 18). Tumor immunity has the following general features: 1. Tumors express antigens that are recognized as foreign by the immune system of the tumor-bearing host. 2. The normal immune response frequently fails to prevent the growth of tumors. 3. The immune system can be stimulated to kill tumor cells and rid the host of the tumor.
Tumor Immunology
Cancer Stem Cells
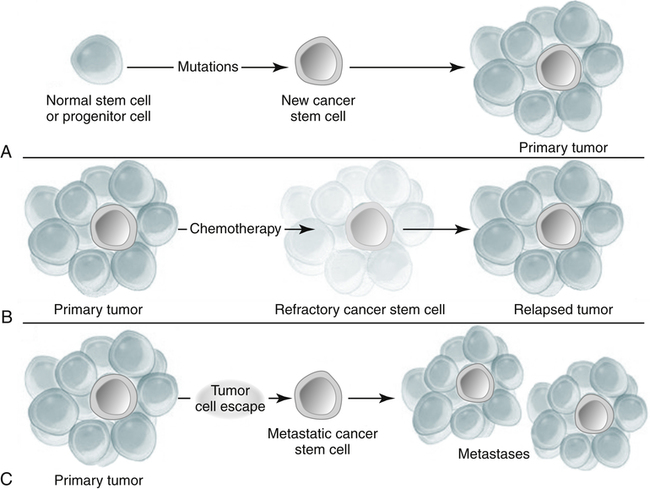
A, Mutation of a normal stem cell or progenitor cell may create a cancer stem cell, which will then generate a primary tumor (Panel A). B, During treatment with chemotherapy, most cells in a primary tumor may be destroyed, but if the cancer stem cells are not eradicated, the tumor may regrow and cause a relapse (Panel B). C, Cancer stem cells arising from a primary tumor may emigrate to distal sites and create metastatic lesions (Panel C). (From Jordan CT, Guzman ML, Noble M: Cancer stem cells, N Engl J Med 355:1253–1260, 2006.)
Types of Tumors
Benign Tumors
Malignant Tumors
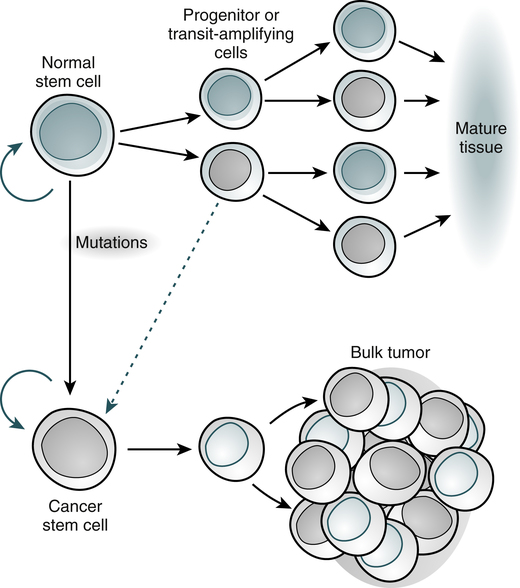
Normal tissues arise from a central stem cell that grows and differentiates to create progenitor and mature cell populations. Key properties of normal stem cells are the ability to self-renew (curved arrow), multilineage potential, and extensive proliferative capacity. Cancer stem cells arise by means of mutation in normal stem cells or progenitor cells and subsequently grow and differentiate to create primary tumors (broken arrow indicates that specific types of progenitors involved in the generation of cancer stem cells are unclear). As with normal stem cells, cancer stem cells can self-renew, give rise to heterogeneous populations of daughter cells, and proliferate extensively. (Adapted from Jordan CT, Guzman ML, Noble M: N Engl J Med 355:1253-1260, 2006.)
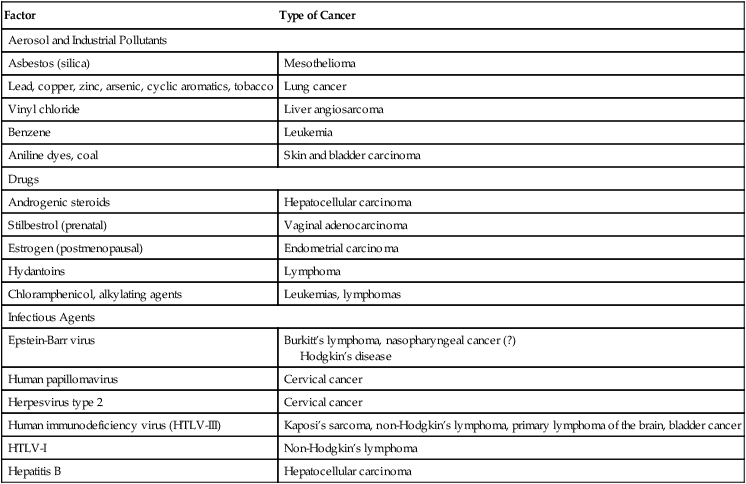
Causative Factors in Human Cancer
Environmental Factors
Factor
Type of Cancer
Aerosol and Industrial Pollutants
Asbestos (silica)
Mesothelioma
Lead, copper, zinc, arsenic, cyclic aromatics, tobacco
Lung cancer
Vinyl chloride
Liver angiosarcoma
Benzene
Leukemia
Aniline dyes, coal
Skin and bladder carcinoma
Drugs
Androgenic steroids
Hepatocellular carcinoma
Stilbestrol (prenatal)
Vaginal adenocarcinoma
Estrogen (postmenopausal)
Endometrial carcinoma
Hydantoins
Lymphoma
Chloramphenicol, alkylating agents
Leukemias, lymphomas
Infectious Agents
Epstein-Barr virus
Burkitt’s lymphoma, nasopharyngeal cancer (?)
Hodgkin’s disease
Human papillomavirus
Cervical cancer
Herpesvirus type 2
Cervical cancer
Human immunodeficiency virus (HTLV-III)
Kaposi’s sarcoma, non-Hodgkin’s lymphoma, primary lymphoma of the brain, bladder cancer
HTLV-I
Non-Hodgkin’s lymphoma
Hepatitis B
Hepatocellular carcinoma
Host Factors and Disease Associations
Disease
Related Cancer
Paget’s disease
Osteogenic sarcoma
Cryptorchidism
Testicular cancer
Neurofibromatosis
Brain tumors, sarcoma
Esophageal webbing
Esophageal carcinoma
Achlorhydria and pernicious anemia
Gastric carcinoma
Cirrhosis
Hepatoma
Cholelithiasis
Gallbladder cancer
Chronic inflammatory bowel disease
Colon cancer
Migratory thrombophlebitis
Adenocarcinoma, especially pancreatic
Myasthenia gravis, pure red cell aplasia, T cell disorder
Thymoma
Nephrotic syndrome
Membranous carcinomas; lymphomas, especially Hodgkin’s
Viruses
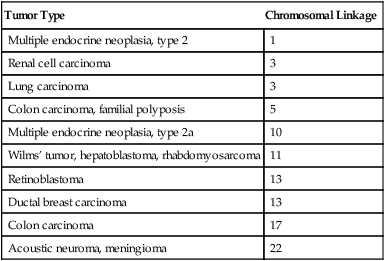
Cancer-Predisposing Genes
Tumor Type
Chromosomal Linkage
Multiple endocrine neoplasia, type 2
1
Renal cell carcinoma
3
Lung carcinoma
3
Colon carcinoma, familial polyposis
5
Multiple endocrine neoplasia, type 2a
10
Wilms’ tumor, hepatoblastoma, rhabdomyosarcoma
11
Retinoblastoma
13
Ductal breast carcinoma
13
Colon carcinoma
17
Acoustic neuroma, meningioma
22
Role of Oncogenes
Oncogene
Disorder
ab1
Chronic myelogenous leukemia
myc
Burkitt’s lymphoma
N-myc
Neuroblastoma
EGFR, HER2
Mammary carcinoma
Ras type
Wide variety of tumors
Mechanisms of Activation
Body Defenses Against Cancer
Tumor Immunology