TNBC Subtype
Immunohistochemistry
Molecular characteristics
Adenoid cystic carcinoma
ER−/PR−/HER2− in >90 % C-KIT+, P63+, EGFR+HCM−, calponin—Ki67 low, TP53—low Topo IIα expression
t(6, 9)(q22–23;p23–24) MYB-NFIB fusion gene
No EGFR gene amplification
No KIT mutation
Metaplastic
ER−/PR−/HER2− in >90 %
Claudin-low associated
Carcinoma
Cytokeratin panel (CK903, AE1/AE3, CK903) variable P63+ (>90 %)
Low expression of GATA3-regulated genes and genes responsible for cell-cell adhesion
Increase in markers linked to stem cell function
Carcinoma with apocrine
ER−/PR− usually HER2− (>50 %)
Gains in 1p, 1q and 2q
Losses of 1p, 12q, 16q, 17q, and 22q19
Differentiation
GCDFP15+ (diffuse)
AR+, BCL2−
Pleomorphic
ER−/PR− (all cases)
Aneuploidy and high S-phase
Carcinoma
HER2− (usually)
Pankeratin+, CAM 5.2+
EMA+ (weak and focal)
P53+ (71 %), Ki67 (high)
BCL2−
Secretory carcinoma
ER−/PR−/HER2−. EMA+, S-100 protein+, E-cadherin+. CK5/6 and 14+
t(12, 15) ETV6-NTRK3 gene fusion. Alteration of the ETV6 gene in both the in situ and invasive components
Carcinoma with medullary features
ER−/PR−/HER2− in >90 %
EGFR gene amplification
CK5/6, EGFR, TP53+ (variable)
TP53 gene mutation
KI-67 high
BRCA1 gene mutations common
Epstein-Barr virus infection?
Basal-like breast
ER−/PR−/HER2− (all cases)
TP53 mutation (83 %)
Carcinoma
CK5/6+, EGFR+ (45–70 %)
Alteration in BRCA-1 activity and loss of function
CK14+, IMP3+, CKIT+ (45 %)
P53+. Others: VEGF+, maspin+
X-chromosome abnormalities
osteopontin, Integrin β4
ID4 and cyclin E1 expression
Caveolin1 and 2+
VEGF and Fascin expression
Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (ACC) of the breast is a rare and morphologically distinct form of breast cancer, comprising less than 1 % of all cases [4]. In contrast to other TNBC, the incidence of mammary ACC among Blacks is significantly lower than in Whites [5]. Histologically, these tumors are identical to their salivary gland counterparts. The tumor is composed of two cell types: cuboidal epithelial cells with rather abundant cytoplasm and pale nuclei lining tubular duct–like structures that contain neutral polysaccharides (PAS positive, diastase sensitive), and myoepithelial–like cells that elaborate acid mucopolysaccharides (alcian blue positive) and abundant basal lamina material. Mammary ACC can assume several architectural patterns including: solid, cribriform, tubular, and trabecular configurations. These patterns may not be distributed homogenously in a given tumor causing a potential diagnostic dilemma, especially on core needle biopsies. A predominant cribriform pattern may be confused with invasive or in situ cribriform carcinoma, and collagenous spherulosis (Fig. 11.1a). DCIS in association with ACC is seen in a minority of cases and may be difficult to distinguish from the surrounding nests of invasive carcinoma.
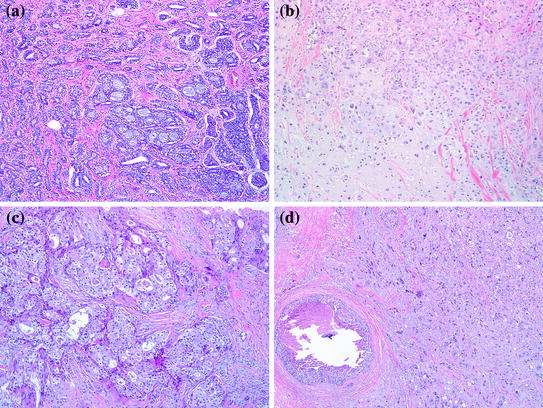

Fig. 11.1
a Adenoid cystic carcinoma, H&E 100×. b Metaplastic Carcinoma with mesenchymal differentiation, 100×. c Carcinoma with apocrine differentiation, 100×. d Pleomorphic carcinoma, 100×
Immunohistochemistry
Immunohistochemistry is helpful in cases of ACC, not only to confirm the diagnosis, but also to differentiate it from its mimickers. A panel including C–KIT (CD117), P63, heavy chain myosin, and calponin is very helpful [6]. ACC is usually positive for C–KIT and P63, and negative for both heavy chain myosin and calponin. Collagenous spherulosis and cribriform DCIS are positive for all myoepithelial cell markers but negative for C–KIT, while invasive cribriform carcinoma will not express any of those markers. Although, typically negative for ER, PR, and HER-2, up to 12 % of mammary ACC have been reported to be ER+/PR+ [5]. Other immunohistochemical studies dedicated solely to mammary ACC are identified in the literature, but are all limited by the small number of cases studied. These studies show a low proliferation index manifested by Ki-67, lack of P53 expression [7], and low Topoisomerase IIα expression, while demonstrating an over-expression of EGFR in 65 % of ACC cases [8].
Molecular Characteristics
Microarray–based gene expression studies have included ACC in the basal-like category due to their triple negative phenotype and expression of basal cell markers. However, studies focusing only on ACC show some molecular differences that distinguish ACC from the crowd of TNBC. ACC consistently displays t(6;9)(q22–23; pp 23–24) translocation, which generates a fusion transcript involving the MYB and NFIB genes [9]. This translocation is considered the key oncogenic mechanism in the pathogenesis of ACC. EGFR gene amplification which has been shown in some basal-like breast cancers, has not been demonstrated in ACC [8]. Similarly, C-KIT expression characteristic of ACC does not reflect an underlying KIT mutation.
Metaplastic Carcinoma
The term metaplastic carcinoma refers to a heterogeneous group of invasive breast carcinoma with microscopic features that diverge from glandular differentiation. These include either squamous or mesenchymal cell differentiation (e.g., spindle cell, chondroid, osseous, or myoid). Metaplastic carcinoma accounts for approximately 1 % of all invasive breast carcinomas. Clinically, patients present in a similar fashion to patients with invasive ductal carcinoma, NOS, in terms of their age at presentation and the manner in which the tumor is detected. The mammographic appearance of metaplastic carcinoma is not specific, except in tumors with osseous metaplasia, where bone-forming areas can be radiologically identified. Microscopically, metaplastic carcinoma varies in the type and extent of metaplastic change. In some tumors, the metaplastic foci may be present as isolated microscopic foci in an otherwise typical invasive ductal carcinoma. In other cases, particularly tumors with squamous and spindle cell metaplasia, the metaplastic component can present in a pure form without any recognizable glandular component. The latter may be difficult to differentiate from a malignant phyllodes tumor or a sarcoma on needle core biopsies. The most common heterologous elements in metaplastic carcinoma are osseous and chondroid differentiation (Fig. 11.1b). In these tumors, the bone and cartilage may appear histologically benign or frankly malignant, further raising the possibility of a sarcoma. The presence of DCIS in tumors with a predominant mesenchymal component, supports the diagnosis of metaplastic carcinoma.
Immunohistochemistry
The diagnosis of metaplastic carcinoma in challenging cases lies in the identification of the epithelial origin of the tumor cells. This may require the use of a panel of low-and high-molecular weight cytokeratins since many metaplastic carcinomas show only focal positivity for CK or may even be negative to some. CK903(34betaE12) and P63 have been reported as sensitive markers for metaplastic carcinomas [10]. As other members of the TNBC family, >90 % of metaplastic carcinomas are negative for ER, PR, and HER2.
Molecular Characteristics
Metaplastic carcinoma are thought to arise from altered epithelial and/or myoepithelial cells. This theory is supported by cytogenetic and molecular studies that demonstrate the same clonality in both the glandular and non-glandular components of the tumor, indicating a common stem cell origin [11, 12]. Collectively, metaplastic carcinomas fall into the category of basal-like breast cancer, however, recent studies suggested that a subset of these tumors displays transcriptomic features consistent with cells undergoing epithelial-to-mesenchymal transition. These are referred to as Claudin-low tumors. Metaplastic carcinomas and claudin-low tumors are shown by comparative genomic hybridization to have low expression of GATA3-regulated genes and of genes responsible for cell-cell adhesion with enrichment for markers linked to stem cell function [13].
Carcinomas with Apocrine Differentiation
Focal apocrine differentiation can be seen in many types of breast carcinoma including: lobular, ductal, tubular, micropapillary, and even medullary carcinoma. However, carcinomas with extensive apocrine differentiation represent approximately 4 % of breast cancer and are represented in this category [14]. Histologically, the majority of the tumor cells display features reminiscent of apocrine cells such as enlarged round nuclei with prominent nucleoli and abundant granular eosinophilic cytoplasm that is PAS-positive (Type A cells) (Fig. 11.1c). Some cells may have abundant foamy cytoplasm, which is referred to as Type B cells, while other tumors may have a combination of both.
Immunohistochemistry
A typical apocrine carcinoma shows diffuse positivity for GCDFP-15 [14], and BCL-2 negativity. Staining for ER, PR is usually negative. A portion of these tumors is also negative for HER2 protein over-expression (triple negative). Androgen receptor expression in ER-negative breast tumors was found to be associated with apocrine differentiation [15].
Molecular Characteristics
The immunophenotypic signature described above has inspired researchers to look for a similar “apocrine molecular signature.” Microarray studies show increased androgen signaling and overlap with the HER2 group of tumors. However, this proposed molecular signature does not correlate well with apocrine morphology. Approximately, only half of carcinomas with apocrine differentiation show this molecular signature. Comparative genomic hybridization has identified several copy number alterations in carcinomas with apocrine differentiation including gains of 1p, 1q and 2q, as well as losses of 1p, 12q, 16q, 17q, and 22q [16]. However, these are also common alteration regions that are seen in breast carcinoma in general. This data suggests that although carcinomas with apocrine differentiation may have a characteristic morphology and immunophenotype, they do not represent a distinct molecular entity.
Pleomorphic Carcinoma
This unusual and rare tumor is considered a variant of high-grade invasive ductal carcinoma, NOS. Morphologically, it is characterized by proliferation of pleomorphic, bizarre cells with greater than sixfold variation in nuclear size. Multinucleated tumor giant cells are common and account for more than 50 % of the tumor cells. Areas of conventional adenocarcinoma may be present. However, pure pleomorphic carcinoma and cases associated with metaplastic carcinoma, especially of the spindle cell type, may be seen and can be misdiagnosed as sarcoma or metastatic tumors. The presence of adjacent foci of ductal carcinoma in situ supports a breast primary in challenging cases (Fig. 11.1d). Axillary lymph node metastases are present in almost half the cases.
Immunohistochemistry
In the original series by Silver and Tavassoli, all tumors showed strong, diffuse positivity for pan-cytokeratin and CAM 5.2, which is useful in differentiating these tumors from sarcomas. EMA was positive in areas of conventional ductal carcinoma, but was very weak and focal in the multinucleated tumor cells. All tumors were negative for ER and PR. HER-2 was also negative in the majority of cases, especially those with node-negative disease [17]. P53 expression was present in 71 %, but none expressed bcl-2. Ki-67 proliferation index was also increased with a mean of 33 %.
Molecular Characteristics
The data on pleomorphic carcinoma is very sparse due to the rarity of these tumors. Nevertheless, the majority of these tumors show aneuploid DNA content and high S-phase.
Secretory Carcinoma
Secretory carcinoma (SC) is an exceptionally rare, low-grade carcinoma accounting for <0.15 % of all breast cancers. They occur over a wide age range, but more commonly in children and young adults “Juvenile carcinoma.” Clinically, they are well-circumscribed tumors, located close to the areola. Grossly, the tumor size ranges from 0.5 to 12 cm with an average of 3 cm. Microscopically, secretory carcinoma has pushing borders and is composed of polygonal cells with granular eosinophilic to foamy cytoplasm (Fig. 11.2a). A consistent finding is the presence of intracellular and extracellular, eosinophilic, secretory material that is positive for PAS and alcian blue (Fig. 11.2b, c). The tumor displays one or more of three growth patterns: solid, tubular, and microcyctic. The latter resembles thyroid follicles. Most tumors contain a mixture of all three patterns.
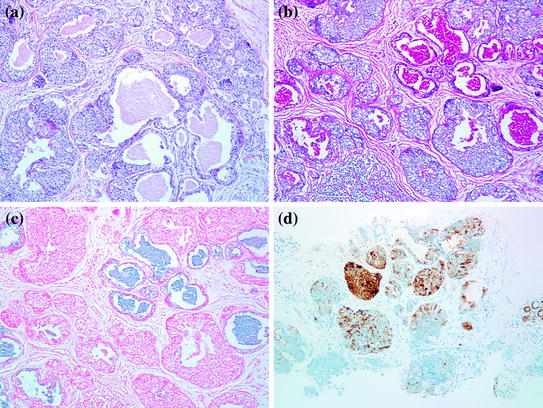

Fig. 11.2
Secretory carcinoma. a H&E, 100×. b Luminal secretions are strongly PAS-D positive. c Alcian blue positive. d S-100 protein is also strongly positive
Immunohistochemistry
The tumor cells are negative for ER, PR, and HER-2, and frequently positive for epithelial membrane antigen (EMA), S-100 protein, and E-cadherin (Fig. 11.2d). Expression of basal cytokeratins (CK 5/6 and 14) was also identified in five out of six cases in one study, suggesting that secretory carcinoma belongs to the basal-like group of breast cancer [18].
Molecular Characteristics
In 2002, Tognon et al. [19] have shown that secretory carcinoma is characteristically associated with t(12, 15) that results in ETV6-NTRK3 gene fusion, the same translocation which was originally described in congenital fibrosarcoma and cellular mesoblastic nephroma. Additionally, FISH analysis shows alteration of the ETV6 gene in both the in situ and invasive components [18].
Carcinoma with Medullary Features
Classic medullary carcinoma (MC) is very rare, representing less than 1 % of all breast cancers. The diagnosis of classic MC requires stringent diagnostic criteria which include histological circumscription, lack of tubular formation with syncytial architecture in >75 % of the tumor, intense lymphoplasmacytic infiltration, and highly pleomorphic tumor cells with numerous mitoses. Tumors that lack some of these features are classified as “atypical medullary carcinoma” or “invasive ductal carcinoma with medullary features.” However, these criteria are often difficult to apply resulting in a high interobserver variability. For the same reasons, the new WHO Classification of Tumors of The Breast has now grouped classic and atypical medullary as well as a subset of invasive carcinoma of no special type under “Carcinomas with medullary features”. Foci of squamous metaplasia can also be seen in MC and should not be considered as a metaplastic carcinoma.
Immunohistochemistry
The majority (>90 %) of MC are negative for ER, PR, and HER-2, with variable expression of basal cytokeratin (CK5/6), EGFR, and P53. The intense lymphocytic infiltrate is predominantly CD3+T lymphocytes. Not surprisingly, MC shows a high proliferation index with Ki-67.
Molecular Characteristics
MC heirs a lot of its molecular features from its basal-like family of breast cancers, including EGFR gene amplification, TP53 gene mutation, and increased incidence in patients with BRCA1 gene mutations [20]. Some questioned the role of Epstein-Barr virus infection in MC given its morphologic similarities with lymphoepithelial carcinomas of other organs. Whereas, one study showed an association between Epstein-Barr virus and MC, another study failed to reproduce this link [21, 22].
Basal-like Breast Carcinoma
Basal-like breast carcinomas (BLBC) is a distinct group of breast carcinoma that has evolved as a separate molecular subtype from gene expression profiling studies [23, 24]. They usually present as rapidly growing breast masses, most probably as “interval breast cancers” (those diagnosed between annual mammograms) [25]. Radiologically, they are often ill-defined, oval, round, or lobulated masses. Extensive necrosis may give the impression of a partially solid and cystic mass on ultrasound. Except for circumscription and geographic necrosis, BLBC shares a lot of histologic features with medullary carcinoma. The tumor is usually grade III invasive ductal carcinoma with focal/absent in situ component, high nuclear grade, absence of tubular formation, and high-mitotic rate. There is usually a dense stromal lymphocytic infiltrate, a solid architecture with pushing borders and areas of geographic necrosis (Fig. 11.3a). Like medullary carcinoma, BRCA-1 associated carcinomas are often BLBC.
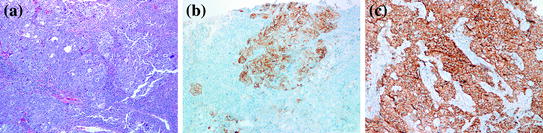

Fig. 11.3
Basal-like breast cancer. a H&E stain, 100×. b CK5/6 immunostain showing positive staining of the tumor cells. c IMP3 immunostain is diffusely positive
Immunohistochemistry
Expression of basal cytokeratins, particularly CK5/6 and CK14 is considered the sine-qua-non of BLBC (Fig. 11.3b). CK17 is also present in approximately 50 % of cases, but may be focal and weak. The expression of EGFR in BLBC varies in several studies, ranging from 45 to 70 %. Since more than 80 % of BRCA-1 associated cancers cluster in the basal-like category, it is not surprising that basal CKs and EGFR expression are also observed in BRCA-1 associated breast cancers. However, attempts to use these markers together with hormone receptors to predict mutation status in these patients has not been successful due to the high overlap between both BRCA-1 and non BRCA-1 associated BLBC [26]. Various other immunohistochemical markers have been studied as a tool to recognize and further characterize this specific subset of tumors. Insulin-like growth factor-II mRNA-binding protein 3 (IMP3), which was first introduced as a marker of aggressive behavior in renal cell and urothelial carcinomas [27], has been demonstrated in 78 % of TNBC, and correlates with CK5/6 expression (Fig. 11.3c) [28]. C-KIT has been reported in approximately 45 % of BLBC. P53 over-expression is also more common in BLBC compared to all breast cancers. Vascular endothelial growth factor (VEGF), maspin, osteopontin, integrin β4, caveolin1 and 2 have all been reported to be preferentially expressed in BLBC [29–33]. However, the only IHC signature of BLBC that has been validated by expression profiling demonstrates that a panel composed of ER, HER2, CK 5/6, and EGFR can identify these tumors with 100 % specificity and 76 % sensitivity [34].
Molecular Characteristics
The literature has been enriched by many studies that focus on better understanding of the molecular background of BLBC, in attempt to translate this molecular phenotype into targeted therapy. TP53 mutation has been identified in up to 83 % BLBC cases. The mutation is thought to be an early event in tumorigenesis and is related to poor prognosis and resistance to chemotherapy [35, 36]. The link with BRCA-1 gene mutation is well established. More than 80 % of BRCA-1 associated cancers cluster in the basal-like category [37], and many sporadic BLBC were shown to have altered BRCA1 activity and loss of function. Approximately 10–20 % of BLBC show methylation of gene promoter, and some have decreased BRCA1 mRNA. X-chromosome abnormalities, including defects in inactivation, were also identified. Additionally, the dominant-negative transcriptional regulator ID4 has been shown to regulate BRCA1 expression and to be preferentially expressed in BLBC [38–40]. Additionally, the loss of one TP53 allele in mice with mammary-specific deletion of BRCA1 dramatically accelerates mammary tumorigenesis, suggesting that TP53 mutations may act synergistically with BRCA1 defects in sporadic BLBC to drive tumor initiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree