Historically the approach to treating noncutaneous melanoma was largely guided by the experience with cutaneous melanoma, particularly in the metastatic setting. However, as genetic tools have allowed clinicians to better characterize these malignancies, their unique biology has become apparent. The ability to accurately distinguish the subtypes of melanoma and the genetic alterations that drive them is beginning to yield the tools that are shifting this disease from one that has proved to be intractable in the advanced setting to one that can be effectively treated.
Key points
- •
Molecular characterization of uncommon subsets of melanoma has revealed that mucosal and ocular melanomas are distinct disease subtypes with unique biologic features that have a direct bearing on treatment.
- •
Surgery, if feasible, offers the best chance for cure in localized ocular and mucosal melanoma. However, in select patients with ocular melanoma, plaque therapy can substitute for enucleation, with similar outcomes.
- •
Although systemic treatment of advanced disease has historically been guided by the experience with cutaneous melanoma, targeted treatments addressing the unique genetics of ocular and cutaneous melanomas are showing significant promise.
Introduction
Biology and Epidemiology
Recent advances in the understanding of melanoma have allowed clinicians to move away from a classification system organized according to histologic differences toward a genetics-based system that has important therapeutic and prognostic implications. One consequence of this is that clinicians are now better equipped to understand and exploit the unique characteristics of melanomas that do not arise from the skin.
The number of cases of skin melanoma in the United States in 2013 was estimated to be 76,690. Among melanomas, 5% to 10% are noncutaneous. Such malignancies can be broadly separated into those arising from the eye and those arising from the mucosal surfaces of the body.
Mucosal melanoma
Approximately 55% of mucosal melanomas (MMs) arise from the head and neck while 24% and 18% arise from the anorectal and vulvovaginal regions, respectively. Melanomas arising from the urinary tract, cervix, esophagus, and gallbladder constitute the remaining 3%.
The epidemiology of MM differs significantly from that of cutaneous melanoma. The median age at diagnosis for MM is 67 years, approximately 1 decade later than for cutaneous disease. Because of the mucosal area associated with the female genital tract, women are approximately 80% more likely than men to be diagnosed with MM, whereas cutaneous melanoma has a slight male predominance. The incidence of MM has remained stable, whereas cutaneous melanomas are being diagnosed with increasing frequency ; moreover, unlike the association between exposure to ultraviolet light and fair skin with cutaneous melanoma, there is no well-established risk factor or race predilection for MM.
Prognostically MM is also unique. Irrespective of stage, the 5-year overall survival of MM is 25%, in stark contrast to the 80% survival at 5 years for cutaneous melanoma. Suggested explanations for poor outcomes with mucosal disease include the challenge of diagnosing MM early in its evolution, and the rich lymphovascular supply at mucosal surfaces. Among the subtypes, vulvovaginal disease has the bleakest prognosis, with only 11.4% surviving at 5 years. 19.8% of patients with anorectal MM and 31.7% of patients with head and neck MM are alive at 5 years. There is no universal staging system for MM, as prognosticators remain elusive. Depending on subtype, nodal involvement and size greater than 3 cm appear to correlate with prognosis in retrospective analyses; prospective validation, however, is lacking.
Approximately half of all melanomas harbor mutations in BRAF, and only 28% are wild-type for BRAF, NRAS, and KIT. Among MMs, however, 55% are wild-type for these oncogenes. One-quarter of MMs harbor mutations in KIT, 12% have mutations in NRAS, and the remaining 9% contain BRAF mutations. The therapeutic implications of these findings are discussed later in this article.
Ocular melanoma
Approximately 95% of ocular melanomas arise from the uvea, including the iris, the ciliary body, and the choroid. Less than 5% arise from the conjunctiva, and less than 1% arise from the eyelid or the orbit. Five percent of all melanomas are ocular, yet most primary ocular cancers in adults are melanomas. Uveal melanoma accounts for 70% of all malignancies arising from the eye.
Approximately 1500 cases of ocular melanoma are diagnosed in the United States annually, and cases occur 30% more frequently in men than in women. Independent risk factors for uveal melanoma are light eye or skin color, nevi of the skin or iris, and possibly exposure to ultraviolet light, including occupational exposures (eg, welding).
Although only 1% of patients harbor distant disease at presentation, approximately half of all patients with ocular melanoma succumb to metastatic disease. The precise site of origin has important prognostic implications. Melanomas arising from the iris have a 10-year survival rate of 95%, whereas ciliochoroidal tumors have a 77% survival rate at 10 years after diagnosis. Tumor size, tumor pigmentation, iris color, and degree of local invasion correlate with poor outcomes.
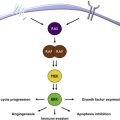
Like MMs, ocular melanomas have unique genetic features that correspond to a distinct biology. GNAQ and GNA11, genes encoding the α subunits of G proteins, are mutated in more than 80% of uveal melanomas, whereas such mutations are present in only 4% of melanomas generally. Such mutations are thought to drive tumor growth by activating downstream growth pathways including the mitogen-activated protein kinase (MAPK) pathway. Mutations in the deubiquitinating gene BAP1, and in codon 625 of splicing factor 3B (SF3B) have also been implicated in the pathogenesis and progression of uveal melanoma.
Introduction
Biology and Epidemiology
Recent advances in the understanding of melanoma have allowed clinicians to move away from a classification system organized according to histologic differences toward a genetics-based system that has important therapeutic and prognostic implications. One consequence of this is that clinicians are now better equipped to understand and exploit the unique characteristics of melanomas that do not arise from the skin.
The number of cases of skin melanoma in the United States in 2013 was estimated to be 76,690. Among melanomas, 5% to 10% are noncutaneous. Such malignancies can be broadly separated into those arising from the eye and those arising from the mucosal surfaces of the body.
Mucosal melanoma
Approximately 55% of mucosal melanomas (MMs) arise from the head and neck while 24% and 18% arise from the anorectal and vulvovaginal regions, respectively. Melanomas arising from the urinary tract, cervix, esophagus, and gallbladder constitute the remaining 3%.
The epidemiology of MM differs significantly from that of cutaneous melanoma. The median age at diagnosis for MM is 67 years, approximately 1 decade later than for cutaneous disease. Because of the mucosal area associated with the female genital tract, women are approximately 80% more likely than men to be diagnosed with MM, whereas cutaneous melanoma has a slight male predominance. The incidence of MM has remained stable, whereas cutaneous melanomas are being diagnosed with increasing frequency ; moreover, unlike the association between exposure to ultraviolet light and fair skin with cutaneous melanoma, there is no well-established risk factor or race predilection for MM.
Prognostically MM is also unique. Irrespective of stage, the 5-year overall survival of MM is 25%, in stark contrast to the 80% survival at 5 years for cutaneous melanoma. Suggested explanations for poor outcomes with mucosal disease include the challenge of diagnosing MM early in its evolution, and the rich lymphovascular supply at mucosal surfaces. Among the subtypes, vulvovaginal disease has the bleakest prognosis, with only 11.4% surviving at 5 years. 19.8% of patients with anorectal MM and 31.7% of patients with head and neck MM are alive at 5 years. There is no universal staging system for MM, as prognosticators remain elusive. Depending on subtype, nodal involvement and size greater than 3 cm appear to correlate with prognosis in retrospective analyses; prospective validation, however, is lacking.
Approximately half of all melanomas harbor mutations in BRAF, and only 28% are wild-type for BRAF, NRAS, and KIT. Among MMs, however, 55% are wild-type for these oncogenes. One-quarter of MMs harbor mutations in KIT, 12% have mutations in NRAS, and the remaining 9% contain BRAF mutations. The therapeutic implications of these findings are discussed later in this article.
Ocular melanoma
Approximately 95% of ocular melanomas arise from the uvea, including the iris, the ciliary body, and the choroid. Less than 5% arise from the conjunctiva, and less than 1% arise from the eyelid or the orbit. Five percent of all melanomas are ocular, yet most primary ocular cancers in adults are melanomas. Uveal melanoma accounts for 70% of all malignancies arising from the eye.
Approximately 1500 cases of ocular melanoma are diagnosed in the United States annually, and cases occur 30% more frequently in men than in women. Independent risk factors for uveal melanoma are light eye or skin color, nevi of the skin or iris, and possibly exposure to ultraviolet light, including occupational exposures (eg, welding).
Although only 1% of patients harbor distant disease at presentation, approximately half of all patients with ocular melanoma succumb to metastatic disease. The precise site of origin has important prognostic implications. Melanomas arising from the iris have a 10-year survival rate of 95%, whereas ciliochoroidal tumors have a 77% survival rate at 10 years after diagnosis. Tumor size, tumor pigmentation, iris color, and degree of local invasion correlate with poor outcomes.
Like MMs, ocular melanomas have unique genetic features that correspond to a distinct biology. GNAQ and GNA11, genes encoding the α subunits of G proteins, are mutated in more than 80% of uveal melanomas, whereas such mutations are present in only 4% of melanomas generally. Such mutations are thought to drive tumor growth by activating downstream growth pathways including the mitogen-activated protein kinase (MAPK) pathway. Mutations in the deubiquitinating gene BAP1, and in codon 625 of splicing factor 3B (SF3B) have also been implicated in the pathogenesis and progression of uveal melanoma.
Clinical management of locoregional disease
Evaluation and Workup
Staging mucosal melanoma
In part owing to the rarity of the disease, staging systems that have been proposed to date, including the American Joint Committee on Cancer (AJCC) staging system for cutaneous melanoma, have failed to reliably separate MM into distinct prognostic categories across subtypes. The Ballantyne staging system, presented in 1970 as a way of staging MMs of the head and neck, has subsequently been used to stage MMs more broadly. This system divides cases into those with clinically localized disease (Stage 1), those with regional lymph node involvement (Stage 2), and those with distant metastases (Stage 3). Its ease of use and straightforward descriptive nature have allowed it to gain acceptance for anorectal and vulvovaginal disease in addition to head and neck MM. However, given that most patients with MM present with localized disease, and that positive lymph nodes are of indeterminate significance, even for MM of the head and neck the practical value of this system is limited.
A more useful method for staging MM takes into account the unique prognostic and therapeutic implications of the site from which the disease stems. A recently proposed staging system from the AJCC specifically for MMs of the head and neck has been shown to predict prognosis. To account for the poor prognosis in even the most limited disease, the lowest possible stage is III and patients can be diagnosed with stage IV disease by local invasion alone, without nodal or distant spread. The key distinction between disease of stages IVA, IVB, and IVC is resectability.
With regard to anorectal disease, the Ballantyne staging system has been shown to correlate with prognosis in a retrospective series. Proposed schema to stage vulvovaginal melanoma, whether adaptations from systems devised for the skin or systems that are specific to the female genital tract, have been unable to consistently predict prognosis. The 2002 modified AJCC system for staging cutaneous melanoma, however, has been shown to predict recurrence-free survival for vulvar melanoma. While much work remains to be done to better understand how best to stage MM, particularly when it arises from the vagina or anorectum, a suggested approach to selecting a staging system that takes into account subtype and avoids unnecessary complexity is presented in Table 1 .
| Subtype | Suggested Staging Method |
|---|---|
| Head and neck | AJCC head and neck system for mucosal melanoma |
| Anorectal | Ballantyne staging system |
| Vulvar | AJCC cutaneous melanoma staging system |
| Vaginal | Ballantyne staging system |
| Cervical | FIGO staging system |
| Urethral | Levine system for urethral carcinoma |
Staging ocular melanoma
Three prognostic systems have a place in uveal melanoma, based on anatomy, cytogenetics, and gene expression profiling, respectively. The 2010 version of the AJCC staging system for uveal melanoma has been demonstrated to accurately predict prognosis by taking into account tumor size and invasion. Cytogenetically, monosomy of the third chromosome has been correlated with a poor prognosis, particularly when found in the setting of alterations in chromosome 8. Finally, a commercially available 15-gene expression profile array has demonstrated excellent prognostic power in a validation cohort.
Treating Locoregional Mucosal Melanoma
Surgery
Although cure rates for localized disease remain dismal, both surgery and radiation are potentially definitive modalities, with surgery typically offering the best chance for cure. However, the use of surgery is frequently limited by the multifocal nature of tumors’ growth and the fact that local recurrence rates are generally greater than 50%. For most localized tumors, anatomic constraints ultimately determine whether surgery is feasible.
For MM of the sinuses or nasal cavity without nodal involvement, wide surgical resection without lymph node dissection with consideration of postoperative radiation therapy to the primary site is advocated. This approach may entail craniofacial resection for tumors involving the cribriform plate, orbital exenteration for orbital involvement, and radical nasal exenteration for diffuse mucosal disease. An endoscopic approach may reduce morbidity and improve functional outcomes. Some consider forgoing radiation for limited tumors that do not involve deep soft tissues, cartilage, bone, or skin.
If regional lymph nodes are involved but the tumor does not involve the brain, masticator space, skull base, dura, carotid artery, cranial nerves IX through XII, or the prevertebral space, the lesion is still considered potentially resectable. In this case a wide surgical resection with neck dissection and postoperative radiation to the primary site and the neck is advocated, based on reports of improvement in local control. For MM of the oral cavity, oropharynx, larynx, or hypopharynx, even if regional lymph node involvement is not suspected, neck dissection is advocated if the primary lesion involves deep soft tissues, cartilage, bone, or skin. More advanced disease can be treated with either radiation or systemic therapy (see later discussion).
Historically, anorectal MMs were managed with abdominopelvic resections, whereas vulvovaginal MMs were treated with anterior pelvic exenteration. Despite the aggressive nature of this surgery and the high associated morbidity, local and distant recurrence rates remained high. For this reason it was hypothesized that wide local excisions may offer similar outcomes without the need for more radical surgery. Although retrospective data suggest that this more restrained approach may increase the rate of local recurrence, it does not appear to confer a worse overall survival, possibly because of the high rate of distant metastasis that frequently determines survival.
The role for lymph node dissection in this setting remains undetermined and, as discussed earlier, nodal status is not an established prognosticator for anorectal or vulvovaginal MM. Furthermore, the presence of distant metastases in the absence of nodal involvement is a well-documented phenomenon.
Radiotherapy
Whereas historical in vitro data suggest that melanoma cells are able to survive sublethal doses of ionizing radiation, more recent data have called these results into question. In fact, some have even reported local control rates for MM as high as 85% with definitive radiotherapy.
A logical extrapolation from these data is that adjuvant radiation can be used to improve local disease control after surgery, and indeed this has been reported. Nevertheless, radiation is yet to show improved overall survival when used after surgery, in part because of the high rate of distant recurrence. This situation may change in light of the fact that optimal dosing schedules, particularly with regard to anorectal and vulvovaginal disease, are still being determined.
Adjuvant chemotherapy
The high rate of recurrence after definitive treatment of primary MM has stimulated great interest in the role of adjuvant systemic treatment. Unfortunately, there has been a paucity of clinical data to guide treatment in this domain. In the only randomized study of adjuvant treatment for MM to demonstrate a survival advantage, Lian and colleagues have shown improved outcomes with a regimen of cisplatin and temozolomide. In this study of 189 patients, those who were randomized to adjuvant temozolomide with cisplatin had an improved overall survival (median 48.7 months) when compared with those who were randomized to surgery alone (21.2 months) or adjuvant treatment with interferon (40.4 months). These results, however, should be interpreted with caution given that melanomas arising in Chinese populations are genetically distinct from those arising in Western patients. Furthermore, it is difficult to reconcile an improved overall survival with the use of chemotherapy in the adjuvant setting when no such benefit has been demonstrated in the metastatic setting. Nevertheless, this study clearly establishes a role for adjuvant chemotherapy in the treatment of resectable MM.
Treating Locoregional Ocular Melanoma
Every effort should be made to optimize the chance for cure of ocular melanoma with first-line treatment, as local recurrence confers an increased risk for metastatic disease. Current approaches to the use of radiotherapy offer outcomes comparable with those of enucleation while sparing the eye, provided that radiotherapy can be initiated promptly after diagnosis. Radiotherapy for ocular melanoma can be divided into 2 categories: brachytherapy, which includes several radioactive plaques containing various radioisotopes, and external beam radiation, which includes charged-particle radiation and photon-based stereotactic radiotherapy.
Radiotherapy: brachytherapy
Radioactive plaques that have been used to treat ocular melanoma include iodine-125 ( 125 I), cobalt-60, iridium-192, palladium-103, and ruthenium 106–rhodium 106. 125 I is often favored, as its off-target effects can be easily shielded while maintaining good tissue penetration. Although higher rates of local recurrence have been reported with plaque therapy in comparison with particle radiation, identifying optimal candidates for brachytherapy may offset this effect. For example, ruthenium plaque therapy has been reported to yield a 2% 5-year recurrence rate for patients with small tumors distal from the optic disc.
Radiation retinopathy is a common adverse effect associated with plaque therapy, reported to occur in 6% of patients at 1 year and 50% of patients at 5 years. Proximity to the optic disc and fovea, in addition to high radiation dose, correlates with the risk of retinopathy. Other side effects of plaque therapy include cataracts, glaucoma, vitreous hemorrhage, and scleral necrosis.
Radiotherapy: external beam radiation
Photon-based external beam stereotactic radiation therapy is an alternative to radioactive plaque therapy. Treatment with this modality is generally administered at a dose of 50 to 70 Gy as 5 daily fractions. Local control at 10 years is approximately 93% for choroidal disease.
Charged particles used to irradiate uveal melanoma include protons, carbon ions, and helium ions. This technique is favored for medium to large choroidal tumors, particularly when they occur near the fovea or optic disc, as this approach provides a sharp decrease in radiation dose outside of the targeted area.
A single-institution study of more than 2000 patients treated with proton therapy reported a local control rate of 95% at 15 years of follow-up, with eye preservation in 84% of patients. Risk factors for visual loss in this experience included tumor location near the optic disc or macula, large tumor size, poor vision at baseline, retinal detachment, and diabetes. A meta-analysis that included 8809 patients showed a significantly lower rate of local recurrence among those who received charged-particle therapy compared with those who received 125 I brachytherapy.
Surgery
Given the success of radiotherapy in controlling localized ocular melanoma while sparing the involved eye, surgery has generally been reserved for cases whereby radiation is not feasible because of large tumor size or extrascleral extension, for example. In 2006, the Collaborative Ocular Melanoma Study Group reported the results of a large randomized study in which patients with choroidal melanoma received either 125 I brachytherapy or enucleation. At 12 years’ follow-up, there was no statistically significant survival benefit for patients randomized to enucleation.
Although enucleation remains the most commonly performed surgery for the treatment of localized ocular melanoma, local resection (eg, with the use of scleral lamellar dissection) continues to be performed for a small subset of patients with ocular melanoma. This procedure has been associated with retinal detachment and vitreous hemorrhage, as well as higher rates of recurrence in comparison with radiotherapy or enucleation. For this reason, such procedures should be performed at high-volume centers, and possibly followed by adjuvant brachytherapy or external beam radiation.
Other approaches
Transpupillary thermotherapy (TTT) is a modality whereby electromagnetic radiation is delivered via an infrared laser to cause tumor necrosis. Complications include vision loss secondary to either maculopathy or retinopathy. Given the superior efficacy of radiotherapy, the use of TTT as definitive treatment for ocular melanoma is not favored; moreover, given its side-effect profile, its use in combination with brachytherapy to improve local control remains questionable.
Photocoagulation involves the delivery of thermal energy to the tumor with the goal of directly lysing melanoma cells and rendering the local vasculature inadequate to sustain the malignancy. Its use had previously been limited to the treatment of small choroidal melanomas; however, owing to its unfavorable side-effect profile including vascular occlusion of the retina, vitreous hemorrhage, and retinal detachment, its use is now limited to a subset of disease that recurs after proton therapy.
Photodynamic therapy using photosensitizing chemicals injected directly into the tumor has also been used. Photosensitive compounds including porfimer and hematoporphyrin, which are activated by light at specific wavelengths, are administered to generate free radicals that damage endothelial cells and induce vascular occlusion to cause tumor necrosis. Results with this method have been discouraging. In one small series, 62% of patients with locoregional disease had recurrent disease within 5 years.
Adjuvant chemotherapy
To date, no adjuvant treatment has demonstrated a survival advantage for patients with ocular melanoma. A trial with maintenance interferon-α2a given over a 2-year period after either enucleation or proton radiation in 121 patients with increased-risk uveal melanoma failed to demonstrate an improvement in overall survival when compared with matched historical controls.
Surveillance
Few objective data exist to guide surveillance after definitive treatment of localized ocular melanoma. However, given that up to 50% of such patients will develop metastatic disease, surveillance is reasonable. Repeat imaging done every 3 to 12 months with particular attention to the liver is reasonable. As magnetic resonance imaging (MRI) is the most sensitive imaging modality for identifying hepatic metastasis, the authors generally use MRI of the abdomen and pelvis together with computed tomography of the chest. Basic blood work including liver function tests may be done on a similar schedule.
The interval of testing can be tailored to risk of recurrence as determined by gene-expression profile, the size of the primary tumor, and histologic findings (eg, degree of plasma-cell infiltration, presence of vasculogenic mimicry). Recent data suggest that inactivating mutations in BAP-1 may also portend a high risk of recurrence, whereas mutations in codon 625 of the splicing factor SF3B1 may confer a more favorable prognosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree