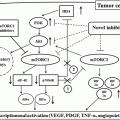
Fig. 6.1
Treatment algorithm for nonmetastatic patients with RCC
6.2.1 Stage 1
6.2.1.1 Surgical Therapy
Surgical therapy remains the mainstream treatment for patients with stage 1 RCC because the resection of primary tumors tends to be curative and provides the most effective oncological outcome [1–3]. Thus, surgical therapy should be considered as the primary treatment option for stage 1 disease. Radical nephrectomy was considered the gold standard of surgical treatment from the 1960s [4]. The original concept of radical nephrectomy was conceptualized by Robson et al., including early ligation of the renal arteries and veins, ipsilateral adrenalectomy, dissection of the kidney outside Gerota’s fascia, and lymphadenectomy [5]. Wide resection of the kidney resulted in improved patient survival compared to simple nephrectomy, the former standard procedure.
However, these results are based on the era when most tumors were found at advanced stages due to the lack of the present radiological modalities. In current practice, however, the proportion of stage 1 disease has increased up to about 70 %, and most of it is found incidentally by radiological screening. Thus, there is controversy about the necessity of wide resection for all RCC tumors. Cumulative results show that ipsilateral adrenalectomy at the time of nephrectomy has no survival benefit; thus, prophylactic adrenalectomy is currently considered unnecessary unless abnormal radiological findings are detected in the adrenal gland [6]. The benefit of lymphadenectomy has been also examined by randomized prospective studies. Although its benefit remains undetermined for advanced stage disease, the EORTC study concludes that lymphadenectomy does not improve survival in patients with low-stage disease [7]. In addition, the disadvantage of radical nephrectomy has been emphasized due to the new onset of chronic kidney disease (CKD) and increased risk of cardiovascular disease and noncancer mortality [8–10]. Thus, the recent preference in surgical treatment for stage 1 disease trends toward partial nephrectomy.
6.2.1.1.1 Radical Nephrectomy or Partial Nephrectomy?
Partial nephrectomy was originally indicated for patients with a solitary kidney, renal dysfunction, or bilateral disease. These patients are not good candidates for radical nephrectomy [11]. But many studies have supported the equivalent oncological outcome between radical and partial nephrectomy [12]. In addition, prevention of CKD has been considered as the great advantage of partial over radical nephrectomy as mentioned above [8–10]. Progression of chronic kidney disease is reported to increase the risk of death, cardiovascular events, and hospitalization in a large community-based population [13]. Whether postoperative CKD really induces cardiovascular events or non-RCC-related death has been debated. Huang et al. reported that radical nephrectomy was significantly associated with a 1.4 times higher risk of more cardiovascular events after surgery compared to partial nephrectomy (p < 0.05) [10]. Population-based studies including large numbers of patients show an increased risk of noncancer mortality with radical nephrectomy as compared to partial nephrectomy despite comparable RCC-related mortality between procedures [9, 14, 15]. However, only one randomized EORTC trial comparing postoperative survival between radical and partial nephrectomy shows similar cancer-specific and overall survival [16]. In the original report, there are several points to be criticized. One is a lack of results on renal function after surgery. Thus, this trial is statistically underpowered. Functional outcome was reported thereafter, and partial nephrectomy resulted in higher postoperative renal function than that after radical nephrectomy [17]. The randomized trial did not show any benefit of partial nephrectomy over radical nephrectomy in reducing the risk of overall survival. Nevertheless, renal function was preserved at a higher level after partial nephrectomy.
There may be several other advantages in partial nephrectomy apart from the reduction of the risk of non-RCC-related death. First, about 5 % of patients develop tumors on the contralateral kidney [18]. If the functioning kidney is preserved during the first surgery, the selection of treatment for contralateral tumors can be more flexible. Second, the proportion of benign tumors is reported to be about 20 % in Western countries [19, 20]. Performing radical nephrectomy on these patients with benign disease may constitute overtreatment. Third, preservation of renal function may allow the patients to undergo systemic therapy for other diseases with agents having renal toxicity, such as cisplatin [21].
Taking these results into consideration, the recommendation of the current guideline is partial nephrectomy for patients with stage 1 disease, especially for T1a tumors whose sizes are 4 cm or less [1, 22, 23]. Radical nephrectomy is considered only when partial nephrectomy is not technically feasible due to the anatomical complexity of the tumors.
Partial nephrectomy should be discussed as a standard care even for tumors of T1b, 4.1–7.0 cm [1]. Most studies support the preservation of renal function after partial nephrectomy [24–26]. However, renal function after partial nephrectomy for T1b tumors is slightly lower than that after nephron-sparing surgery for T1a tumors [26]. The advantage in overall survival from partial nephrectomy is even more unclear [26, 27]. Nephron-sparing surgery does have other advantages over radical nephrectomy as mentioned before. Thus, partial nephrectomy also should be considered for T1b tumors when technically feasible.
There has been controversy over which is the better procedure in terms of nephron-sparing surgery—partial nephrectomy or tumor enucleation. In partial nephrectomy, normal kidney margin is attached to the surroundings of the tumors. This concept is based on possible satellite lesions around the tumors. The incidence of satellite lesions has been reported to be 6–15 % within 1 cm from the tumor boundary [28, 29]. Recent studies, however, show no significant correlation between the thickness of normal kidney margin and local recurrence [30], and the incidence of local recurrence is likely to be very low at 1–2 % [31]. In tumor enucleation, tumors are excised without a margin of normal parenchyma [32, 33]. Tumor enucleation has some advantage in the maximum preservation of functional renal parenchyma and a low incidence of major bleeding and collecting system damage, thereby decreasing the incidence of complications such as urinoma and urinary fistula [33]. In addition, oncological outcomes are reported to be comparable between tumor enucleation and partial nephrectomy [34]. The incidence of positive surgical margin was not different between the procedures (0.2 % versus 3.4 %). Thus, either procedure can be considered acceptable.
Figure 6.2 shows the sequential change by year in the utilization of partial nephrectomy at Tokyo Women’s Medical University. Partial nephrectomy has been selected for about 95 % of patients with T1a disease in recent years. Radical nephrectomy for T1a disease is considered only when the patients are over 80 years old, have CKD stage 4 or 5, and are likely to develop end-stage kidney disease soon even after nephron-sparing surgery [35]. The proportion of partial nephrectomy for T1b tumors has increased over the years and reached up to 90 % in the year 2013. Thus, the more the technique improves for partial nephrectomy, the more we can extend the indication of the nephron-sparing procedure.
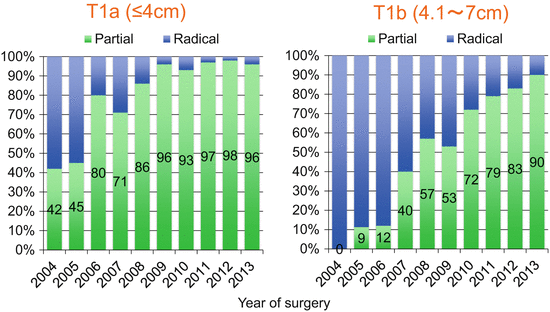

Fig. 6.2
Sequential change of the utilization of partial nephrectomy for stage I tumors at Tokyo Women’s Medical University
6.2.1.1.2 Open, Laparoscopic, or Robotic Surgery?
How do we select the approach—open, laparoscopic, or robotic procedure? According to the current guidelines, the standard procedure for partial nephrectomy is open surgery since laparoscopic partial nephrectomy (LPN) is associated with a higher incidence of complications and longer ischemic time (Table 6.1) [1]. However, recent technical innovations—including early unclamping, zero ischemia, and the accumulation of experience with LPN—have resulted in better residual renal function and lowered the incidence of postoperative complications [36, 37]. Based on these results, the current guidelines allow experienced surgeons to perform LPN [22]. In addition, robot-assisted laparoscopic partial nephrectomy (RAPN) has widely spread around the world [38]. The new technology of robotic partial nephrectomy has made suturing and excising easier and more precise compared to the laparoscopic procedure. Meta-analysis showed an equivalent cancer control and safety of RAPN to LPN and the advantage of RAPN in shortening ischemic time over LPN [39]. Thus, RAPN is more feasible and likely to be indicated for more patients with stage 1 than LPN.
Table 6.1
Recommended approach for surgical treatment by clinical stage
Clinical stage | Procedure | Approach | Comment |
|---|---|---|---|
T1 | Nephron-sparing surgery | Open | Recommended |
Laparoscopic | Optional for experienced centers only | ||
Robot assisted | |||
Radical nephrectomy | Laparoscopic | In patients not suitable for NSS | |
Open | Optional in patients not suitable for NSS | ||
T2 | Radical nephrectomy | Laparoscopic | Recommended |
Open | Optional | ||
Nephron-sparing surgery | Open | Alternative at experienced centers | |
T3, T4 | Radical nephrectomy | Open | Recommended in most patients |
Laparoscopic | Feasible in selected patients |
Laparoscopic radical nephrectomy has shown a significant benefit over open radical nephrectomy with its reduction in intraoperative bleeding, complication rate, and shorter hospital stay [40]. Laparoscopic radical nephrectomy shows equivalent long-term oncological outcome to open radical nephrectomy [41]. Thus, laparoscopic radical nephrectomy is more preferable for stage 1 disease than open radical nephrectomy (Table 6.1) [22].
6.2.1.2 Thermal Ablation
Cryoablation and radiofrequency ablation (RFA) are two less invasive treatment options for small renal masses than surgery. Patients who are not surgical candidates can be still treated with a percutaneous approach under local anesthesia. However, there is a limitation in terms of tumor location for RFA. The most serious complication of RFA is ureteric stricture, which occurred at 3 % [42]. Thus, RFA is not recommended for central tumors located in the hilum, near the proximal ureter, or the central collecting system [22]. Cryoablation has fewer limitations for tumor location compared to RFA. However, percutaneous cryoablation is often found to be time-consuming and shows higher risk of complications about 20 %, with most involving hemorrhagic-related complications including hematoma and requirement of transfusion [43]. Local recurrence after thermal ablation therapy is seen much more frequently than after surgical therapy, 7.4 times higher after cryotherapy, and 18.2 times higher after RFA [44]. The long-term oncological outcomes of cryoablation and RFA are reported to be comparable to that of partial nephrectomy [45, 46].
One major advantage of thermal ablation is its superior preservation of renal function after the procedure. Residual renal function is reported to be higher in cryoablation than that in robotic partial nephrectomy [46, 47]. But, other researchers stated no difference between groups in long-term follow-up [48]. RFA shows better functional outcome than open partial nephrectomy [49], but this advantage decreases when compared to laparoscopic partial nephrectomy [50]. Currently, there is no definitive conclusion as to the superiority of one procedure over the other [51, 52].
Thus, patient selection is important for treatment. A recommended indication for thermal ablation is small renal masses in elderly patients, or those with severe comorbidity, multiple tumors of hereditary background, and bilateral tumors, and patients with a solitary kidney who are at high risk of complete loss of renal function by surgery [22].
6.2.1.3 Active Surveillance
Active surveillance is one of the therapeutic options for small renal masses, since tumor growth is generally slow [53]. The mean linear growth rate of RCC tumors has been reported to be 0.1–0.3 cm per year [54, 55]. In addition, delayed intervention is not associated with increased surgical morbidity and does not compromise oncological outcome [56]. Surgical intervention may have no impact on the prolongation of overall survival of patients of 75 years or older [57]. The most problematic risk from active surveillance is the progression to metastatic disease. Recent studies show the surprisingly low risk of developing metastatic progression of small renal masses (1–2 %) [58]. Thus, the current guidelines recommend that active surveillance should be discussed if patients wish to avoid surgical treatment or are not surgical candidates due to age or severe comorbidities [1, 3, 22]. The recognition of the feasibility of active surveillance has increased the proportion of active surveillance for small renal masses up to about 20 % in a population-based study [59]. However, it should be noted that small renal masses include about 20 % high-grade tumors [60]. Thus, patient selection is important for this option.
6.2.1.4 The Role of Percutaneous Biopsy
Percutaneous biopsy for small renal masses has been increasingly performed during recent years, but its use is still uncommon. One population-based study shows that only 6 % of the patients who underwent surgery for small renal masses underwent a preoperative tumor biopsy [61]. This may reflect the current thought that tumor biopsy is not accurate enough (at 5 % false-positive and 25 % false-negative results) to warrant a change in treatment strategy [62]. However, previous studies included fine needle aspiration which is likely to be inferior in diagnostic accuracy to core biopsy [63]. Fine needle aspiration is not recommended for current practice [64].
Lately, the accuracy of core biopsy is very high. A recent literature review summarizes studies analyzing the diagnostic accuracy of percutaneous core biopsies [65]. The accuracy of differentiating malignant from benign tumors is reported to be 86 %–100 %. Sensitivity and specificity are reported to be 86 %–100 % and 100 %, respectively. Accuracy of core biopsy is 86–98 % in histological subtyping and 46–76 % in grading. The rate of accurate grading is not so high, but this may reflect the heterogeneity of RCC tumors or the interobserver variability in grading by pathologists [64]. Detecting high-grade tumors on biopsy may impact the treatment choice in which extirpative surgery is more preferable than active surveillance or thermal ablation.
Complications of needle biopsy include tumor seeding, bleeding, arteriovenous fistula, infection, and pneumothorax [63]. The incidence of complication for needle biopsy has been reported to be about 5 % [66]. Significant complications which required active treatment or hospital admission were observed at less than 2 % in the recent series [65]. Most of these are bleeding. One of the most concerning complications is tumor seeding, but the estimated risk for this is less than 0.01 % [67]. No tumor seeding was observed in the recent series [65]. Thus, morbidity related to biopsy is low [64]. In addition, biopsy does not complicate the surgery [68].
Indication of biopsy is shown in Table 6.2 [64, 65]. In the setting of thermal ablation, tumor biopsy is strongly recommended before the procedure. This is because pathological interpretation is difficult after treatment due to possible necrosis or degeneration of the tumors. Biopsy is recommended for patients considered for active surveillance (with the possibility of delayed treatment), without clear indication of pathological diagnosis on imaging appearance, or suspicions of metastatic disease. Patients are not recommended to undergo biopsy when the following conditions are met: (1) syndrome with monomorphic pathology; (2) imaging characteristics of specific pathology; (3) conservative management is not an option, in other words, when up-front extirpative surgery is preferred; or (4) watchful waiting without consideration of further active treatment.
Table 6.2
Indications for tumor biopsy for small renal masses
Recommendation | Condition |
|---|---|
Strongly recommended before ablation | In the setting of ablation (before ablation) |
Recommended | Active surveillance (possibility of delayed treatment) |
Without clear indication of pathological diagnosis on imaging appearance | |
Suspicious of metastatic disease | |
Not recommended | Syndrome with monomorphic pathology |
Imaging characteristics of specific pathology | |
Up-front extirpative surgery is preferred | |
Watchful waiting without consideration of further active treatment |
6.2.2 Stages 2 and 3
6.2.2.1 Surgical Therapy
Partial nephrectomy is difficult to perform in most cases for T2 tumors larger than 7 cm, although the results from high-volume centers show an equivalent cancer control between partial and radical nephrectomy for T2 tumors [69, 70]. However, these studies are retrospective, and highly selected patients underwent partial nephrectomy. Thus, partial nephrectomy is only feasible in experienced centers for selected patients, and radical nephrectomy is indicated for most patients [22, 23]. Laparoscopic radical nephrectomy is a recommended procedure for T2 disease (Table 6.1) [22]. Open radical nephrectomy is also recommended, but it shows larger blood loss and longer hospital stay compared to laparoscopic radical nephrectomy [71, 72].
As for T3 or T4 disease, open radical nephrectomy is a standard procedure [22, 23]. Patients with tumor extension to the inferior vena cava or those with invasion to adjacent organs are definite candidates for open procedure. It has been reported that IVC thrombectomy with robotics was successfully performed in selected cases, but the number of cases reported has been still very low [73, 74]. When patients have tumor extension to the right atrium, long-term survival is possible to obtain with radical nephrectomy combined with tumor thrombectomy, which is usually performed under cardiopulmonary bypass [75]. Laparoscopic radical nephrectomy is feasible for selected patients since many reports from experienced centers show equivalent oncological outcome and superior perioperative profile to open radical nephrectomy [76]. It remains undetermined whether partial nephrectomy for T3a tumors is justified in terms of cancer control. Partial nephrectomy is unlikely to compromise the oncological outcome for pathological T2 or T3 tumors which were primarily diagnosed as clinical T1b by preoperative radiological findings [77]. Thus, partial nephrectomy is considered only for patients with a solitary kidney or with bilateral tumors of clinical stage T3 or higher.
6.2.2.2 Lymphadenectomy
One randomized prospective trial shows no therapeutic benefit from lymphadenectomy in the patients with RCC [7]. Pathological lymph node metastases were only found in 3.8 % of the clinically node-negative patients who underwent lymphadenectomy. This indicates that RCC tumors infrequently metastasize to the lymph nodes. This study, however, included only 28 % stage T3 tumors. In other words, the majority of the patients were at a lower stage. Thus, the role of lymphadenectomy in advanced disease was not clarified by this study. Blute et al. reported, on the other hand, that pathological grade 3 or higher, sarcomatoid component, tumor size at 10 cm or larger, pathological T stage at 3 or higher, and presence of tumor necrosis were the risk factors that predict lymph node metastases [78]. If patients meet two or more of these factors, the incidence of lymph node metastases exceeded 20 % [77]. Thus, lymphadenectomy may be beneficial in advanced stage, but no definitive recommendation has been drawn. Lymphadenectomy, however, should be performed to make an accurate staging if the radiological study shows lymphadenopathy [3, 22].
6.2.2.3 Ipsilateral Adrenalectomy
Ipsilateral adrenalectomy was originally advocated by Robson et al. when the modalities of radiological examination were less diagnostic than those of the contemporary era [5]. Several studies examined the risk factors of adrenal metastases, and patients with upper pole tumors larger than 7 cm had been reported to be candidates for adrenalectomy [80]. However, recent retrospective studies show no therapeutic benefit of ipsilateral adrenalectomy as a prophylactic measure. Kutikof et al. reported that the incidence of pathological adrenal metastases was only at 4.4 % in patients with tumors 7 cm or greater [81]. Upper pole location was not a predictive factor. Weight et al. also reported that synchronous adrenal metastases were found only in 2.2 % of the patients who underwent ipsilateral adrenalectomy [6]. Both studies show that risk of metastases to the contralateral adrenal glands does not decrease even after ipsilateral adrenalectomy [6, 81]. It should be noted that most patients with adrenal metastases showed abnormal findings on their adrenal gland on preoperative radiological examination, and incidental metastases to the adrenal glands were rarely observed. Thus, the recommendation of the current guideline is not to perform adrenalectomy unless radiological examination shows a possibility of metastatic lesions in the adrenal glands [22].
6.2.2.4 Adjuvant Therapy
Immunotherapy did not prolong survival after surgery [82]. Several ongoing clinical trials are examining adjuvant-targeted therapy. Recently, the results of the ECOG-ACRIN E2085 phase 3 trial were reported. [83] Patients with a high risk of recurrence were randomly assigned to three treatment groups, including sunitinib, sorafenib, and placebo, and were treated for 54 weeks after surgery. Median disease-free survival was 5.8 years (IQR 1.6–8.2) for sunitinib (hazard ratio [HR] 1.02, 97.5 % confidence interval [CI] 0.85–1.23, p = 0.8038), 6.1 years (IQR 1.7—not estimable [NE]) for sorafenib (HR 0.97, 97.5 % CI 0.80–1.17, p = 0.7184), and 6.6 years (IQR 1.5—NE) for placebo. The conclusion of this trial is that adjuvant treatment with the VEGF receptor tyrosine kinase inhibitors sorafenib or sunitinib showed no survival benefit compared to placebo. In contrast, another randomized phase 3 trials (S-TRAC) of adjuvant sunitinib or placebo for very high risk patients shows the prologation of disease free survival in sunitinib group than that in placebo group (6.8 versus 56. years, HR:0.76; p = 0.03). [84] Therefore, the role of VEGF receptors tyrosine kinase inhibitors as adjuvant setting is still controversial.
6.2.2.5 Other Treatment Options
No alternative options to surgical therapy are recommended for stage 2 or 3 disease since cancer mortality is speculated to be higher than noncancer mortality in stages 2 and 3 [85]. There is no evidence to support a survival benefit from transarterial embolization or systemic therapy for nonmetastatic patients [22].
6.3 Metastatic Patients (Stage 4) (Fig. 6.3)
The treatment algorithm for patients with metastatic RCC is shown in Fig. 6.3. The patients are divided into two groups: those who have metastatic disease at the time of diagnosis of RCC and those who develop metastases after a disease-free interval following surgery. A detailed explanation for this algorithm is described below.
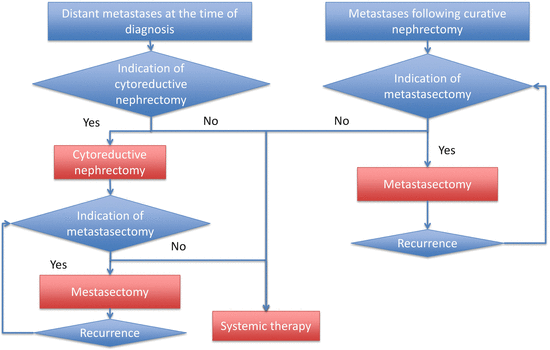

Fig. 6.3
Treatment algorithm for patients with metastatic RCC
6.3.1 Metastatic Disease at the Time of RCC Diagnosis
6.3.1.1 Cytoreductive Nephrectomy
The most important thing to be considered is whether the removal of primary renal tumors by cytoreductive nephrectomy (CN) would be a benefit for patients with metastatic disease. The benefit of CN was established from randomized prospective results during the cytokine era when CN followed by interferon-alpha (IFN-α) significantly prolonged patient survival compared to IFN-α treatment without CN (Table 6.3) [86–88].
Table 6.3
Results of cytoreductive nephrectomy in the cytokine era from two prospective randomized studies
Authors | Number of patients | 50 % overall survival(months) | Objective response rate (%) | Mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
IFNα alone | CN+IFNα | P | IFNα alone | CN+IFNα | P | ||||
SWOG8949 | Flanigan 2001 | 241 | 8.1 | 11.1 | 0.05 | 3.3 | 3.6 | NS | 1 (0.8 %) |
EORTC30957 | Mickisch 2001 | 85 | 7 | 17 | 0.03 | 12 | 19 | 0.38 | 1 (2.4 %) |
Combined analysis | Flanigan 2004 | 331 | 7.8 | 13.6 | 0.002 | 5.7 | 6.9 | 0.60 | 2 (1.4 %) |
The role of CN remains to be determined in the era of targeted therapy. Currently, a randomized prospective study (the CARMENA study) is in process that is comparing the results of a group receiving CN followed by sunitinib to another receiving sunitinib without CN. [89] Thus, there is as yet no determinant evidence to support the role of CN in the era of targeted therapy. It is important to note, however, that the retrospective study demonstrates that CN still has an additional benefit in prolonging patient survival compared to systemic therapy with targeted agents alone [90, 91]. Choueiri et al. reported that their CN group showed a longer median overall survival of 19.8 months than that of their no CN group (19.8 versus 9.4 months, p < 0.01) [90]. The results from the International Metastatic Renal Cell Carcinoma Database Consortium show prolongation of overall survival in patients with CN compared to those without CN (20.5 versus 9.5 months, p < 0.001) [91]. The results from our institution are very similar to their report. The combination with CN prolonged overall survival to 17.1 months from the 8.5 months observed in patients treated with targeted therapy alone (p < 0.0001). CN significantly reduced the risk of patient death after adjusting patients’ risk with the criteria proposed by Heng et al. [92]. Thus, cytoreductive nephrectomy still has a role in the era of targeted therapy [22, 23].
It is important to understand which patients are more likely to benefit from CN. Table 6.4 shows possible prognostic factors influencing the survival of those who undergo CN in conjunction with targeted therapy. The retrospective study shows poor risk in the risk classification system proposed by Memorial Sloan Kettering Cancer Center (MSKCC), 70 % or lower in Karnofsky performance score, the advanced age of 75 years or older, and brain metastases as significant prognostic factors [90]. The degree of reduction of tumor burden by CN is also a prognostic factor. [93] Patients show longer survival when CN removes more than 90 % of the tumor burden. These factors are not contraindications for CN, but they should be taken into consideration when deciding whether CN should be performed.
Table 6.4
Predictive factors for unfavorable outcome after cytoreductive nephrectomy in the era of targeted therapy
Poor risk by risk classification |
Karnofsky performance score of 70 % or less |
Patients of 75 years or older |
Brain metastases |
Degree of reduction in tumor burden by CN less than 90 % |
After CN, systemic therapy and/or metastasectomy should be considered. Details regarding this are described in the following section.
6.3.2 Metastatic Disease After Disease-Free Interval Following Surgery
Some patients develop metastases after prior nephrectomy followed by a disease-free interval. The treatment algorithm is shown in Fig. 6.3.
6.3.2.1 Indications for Metastasectomy
Initially, resectability of metastatic lesions (metastasectomy) should be considered. There are no randomized trials examining the survival benefit of metastasectomy. However, the many retrospective studies show longer survival in patients who received metastasectomy than those without [94–96]. Although the grade of recommendation is not high in the current guidelines because of the lack of randomized trials (grade C), metastasectomy is recommended when metastatic lesions are resectable and the patient has a good performance status [22, 23].
Prognostic factors have been examined to help predict poor prognosis after metastasectomy. Table 6.5 shows the prognostic factors and risk stratification reported by recent representative studies. Naito et al. collected data from 556 patients and report four risk factors that predict poor outcome: grade 3 at the primary tumor, brain metastases, C-reactive protein level higher than 1.0 mg/dl, and incomplete resection [95]. The overall survival of favorable risk patients who meet 0 or 1 factor is 105.6 months, whereas that of patients with 3 or more factors is only 10.3 months. Tosco et al. analyzed the results from 109 patients and report the following five predictive factors: T stage 3 or higher, Fuhrman grade 3 or higher, disease-free interval of 12 months or shorter, non-pulmonary metastases, and multiple site metastases [96]. The overall survival of patients with 0 or 1 factors is more than 108 months, but for those with four or more factors is only 13 months. Alt el al. included 887 patients in their analysis and reported risk factors for poor prognosis after metastasectomy, such as incomplete resection, ECOG-PS 1 or higher, non-pulmonary metastases, and synchronous metastases to nephrectomy [94]. They did not perform risk stratification, but they emphasized complete resection as an important factor for selecting patients for metastasectomy. Eggener et al. reported that patients with poor risk by the MSKCC risk classification show minimum impact from metastasectomy [97]. Although there are no clear criteria for indicating metastasectomy, these factors need to be considered before performing one.
Table 6.5
Risk factors for predicting poor prognosis for metastasectomy and risk stratification
Naito 2013 Urology | Tosco 2013 Eur Urol | Alt 2011 Cancer | ||||
|---|---|---|---|---|---|---|
Patient number | 556 | 109 | 887 | |||
Risk factors | Grade 3 | T stage ≥3 | Incomplete resection | |||
Brain metastases | Fuhrman grade ≥3 | ECOG-PS ≥ 1 | ||||
CRP >1.0 mg/dl | Disease-free interval ≤12 months | Non-pulmonary metastases | ||||
Incomplete resection | Non-pulmonary metastases | Synchronous metastases to nephrectomy | ||||
Multiple sites | ||||||
Risk stratification | 50 % OS (months) | 50 % OS (months) | 50 % OS (months) | |||
Favorable (0, 1 factors) | 105.6 | 0, 1 factors | >108 | Complete resection | 59 | |
Intermediate (2 factors) | 42.0 | 2 | 91 | Incomplete resection | 31 | |
Poor (3 or more factors) | 10.3 | 3 | 38 | No metastasectomy | 13 | |
4 or more | 13 | |||||
We need to keep in mind that these results are based on data from patients treated during the cytokine era. It remains to be determined whether metastasectomy is still beneficial to prolong patient survival during the targeted therapy era since there have been few reports supporting the benefit of metastasectomy. In our institute, 53 patients underwent metastasectomy after 2008 when the first agent of molecular-targeted therapy was introduced in Japan. Complete resection was performed in 43 patients and incomplete resection in ten. One hundred twenty-one patients were treated with systemic therapy alone. Figure 6.4 shows overall survival of patients with metastatic RCC after treatment for metastatic disease. Four-year overall survival of patients with complete resection is 82.2 %. This is significantly higher than that for those with incomplete resection (46.8 %) or no metastasectomy (19.4 %) (p < 0.001). These results are similar to those reported by Alt et al. who show incomplete resection for patients with multiple metastases provided longer survival than that of patients with no metastasectomy [94]. Thus, metastasectomy is likely to be beneficial even in the era of targeted therapy. This is also supported by results from Naito et al. who report that patients receiving metastasectomy and targeted therapy showed prolonged survival as compared to those not receiving targeted therapy (132.2 versus 74.1 months) [95]. Collective results also support the use of metastasectomy when we treat patients with metastatic RCC. However, when patients meet the conditions related to poor prognosis after metastasectomy or when the metastatic lesions are not considered resectable, systemic therapy should be the first choice.
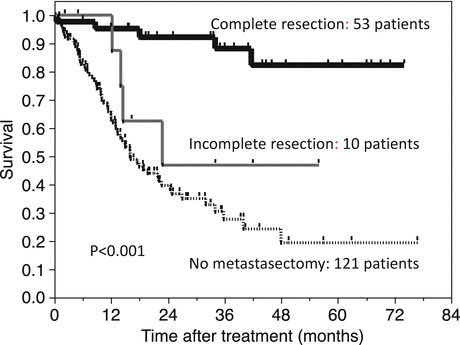

Fig. 6.4
Overall survival of patients with metastatic RCC in the targeted therapy era
6.3.2.2 Systemic Therapy
Systemic therapy is mainstream for the treatment of patients with metastatic RCC. If patients are unlikely to benefit from metastasectomy, systemic therapy is initiated. Stratification of patients with the risk classification system proposed by Memorial Sloan Kettering Cancer Center (MSKCC) is necessary before starting treatment (Table 6.6). The MSKCC risk classification consists of five factors to predict poor prognosis, including time from initial RCC diagnosis to the start of systemic therapy of less than 1 year, low Karnofsky performance status (less than 80 %), hemoglobin of less than the lower limit of normal, corrected serum calcium higher than the upper limit of normal, and lactate dehydrogenase higher than 1.5 times the upper limit of normal [98]. This classification system is based on results from patients treated with interferon-alpha, but is still useful in today’s era of targeted therapy. Heng et al. proposed another risk classification system for patients treated with targeted therapy [92]. This classification consists of the following six variables: time from diagnosis to treatment of less than 1 year, Karnofsky performance status less than 80 %, hemoglobin less than the lower limit of normal, corrected calcium greater than the upper limit of normal, neutrophils greater than the upper limit of normal, and platelets greater than the upper limit of normal. If patients meet no risk factor, they are designated as favorable risk, one to two factors as intermediate risk, and three or more as poor risk. Both classification systems are effective in stratifying patients.
Table 6.6
Risk classification systems for patients with mRCC
MSKCC classification | Heng classification |
|---|---|
(1) Time from initial RCC diagnosis to start systemic therapy: less than 1 year | |
(2) Low Karnofsky performance status: less than 80 % | |
(3) Hemoglobin: less than the lower limit of normal | |
(4) Corrected serum calcium: higher than the upper limit of normal | |
(5) Lactate dehydrogenase: higher than 1.5 times the upper limit of normal | (5) Neutrophils: greater than the upper limit of normal |
(6) Platelets: greater than the upper limit of normal | |
The number of positive factors and classification | |
0 | Favorable risk |
1–2 | Intermediate risk |
3 or more | Poor risk |
Another factor to be determined before starting systemic therapy is histological subtype. If prior nephrectomy has not been performed at the time of systemic therapy and if cytoreductive nephrectomy is not indicated because of poor patient condition, tumor biopsy should be considered. Histological subtype influences the choice of agents.
Table 6.7 shows the currently accepted algorithm based on evidence from clinical trials [99]. For treatment of naïve patients with clear cell histology and good/intermediate risk, standard treatment includes sunitinib, bevacizumab plus interferon-alpha, and pazopanib [100–103]. The use of cytokines, including high-dose interleukin-2 or sorafenib, is an alternative treatment option. Thus, these second options can be used when patients are unfit for the standard option. When patients are at poor risk, temsirolimus is recommended as standard care [104]. Sunitinib can be used as an alternative.
Table 6.7




The currently proposed algorithm for treating patients with metastatic RCC at the year of 2016
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree