Treatment Options for Triple-Negative Breast Cancer
Katherine Enright and Rebecca Dent*
 ABSTRACT
ABSTRACT
The use of modern genomic techniques has significantly enhanced our understanding of breast cancer biology. Five distinct molecular subgroups of breast cancer have been defined and include the hormone receptor-positive luminal A and B, human epidermal growth factor receptor 2 (HER2) positive, “normal” like, and basal like. The last group is frequently identified by immunohistochemistry and commonly referred to as “triple-negative” breast cancer because it lacks expression of both the estrogen and progesterone receptors, as well as HER2. It is characterized by distinct molecular, histologic, and clinical features, including an unfavorable prognosis despite increased sensitivity to cytotoxic therapy. Patients with triple-negative breast cancer do not respond to endocrine agents or trastuzumab and are mainly treated with chemotherapy. Triple-negative breast cancer is heterogeneous and subgroups such as BRCA1 mutation carriers may have a particular sensitivity to platinum-based chemotherapy. The poly (ADP-ribose) polymerase inhibitors appear to be among the most promising “targeted” treatments under investigation for BRCA-associated and triple-negative breast cancer. This article reviews the current treatment strategies for triple-negative breast cancer.
Keywords: breast cancer, triple negative, basal, therapeutics, chemotherapy
Breast cancer is a heterogeneous disease that encompasses a broad spectrum of biological and clinical behaviors. The traditional classification of breast cancer on the basis of hormone receptor expression and the overexpression of human epidermal growth factor receptor 2 (HER2) provides both prognostic and predictive information that help guide clinical practice. The expression of either estrogen receptor (ER) or progesterone receptor (PR) is associated with a favorable outcome and predicts for response to endocrine therapies such as tamoxifen or aromatase inhibitors (1). The overexpression of HER2 is associated with an increased risk of relapse and decreased overall survival (OS) (2). Much of the negative prognostic significance of HER2 overexpression has been negated by the introduction of the targeted anti-HER2 drug trastuzumab (3, 4).
Triple-negative breast cancer (TNBC) comprises 15% to 20% of breast cancers and does not express ER, PR, or HER2 (5). The management of TNBC remains a challenge. Both the TNBC and the HER2-positive subgroups have decreased disease-free survival (DFS) and OS compared with the other breast cancer subtypes (6–10). Unlike HER2-positive breast cancer, TNBC lacks an identifiable therapeutic target. To date, chemotherapy remains the mainstay of treatment for patients with TNBC in the adjuvant and metastatic setting. An increased understanding of the biology of TNBC over the past few years has led to the development of several promising targeted therapeutic strategies that may translate to improved outcomes for this high-risk group. This article will review the current and future strategies for the management of TNBC.
 UNDERSTANDING TNBC
UNDERSTANDING TNBC
Basal-Like Breast Cancer Versus TNBC
Gene expression profiling has provided further insight into the biology of breast cancer and allowed for a more detailed classification of breast cancers. In a landmark paper, Sorlie et al. describes five intrinsic molecular subgroups, including luminal A, luminal B, HER2-enriched, “normal”-like, and basal-like breast cancer (BLBC) (11–13). Compared with the highly estrogen-sensitive luminal A subgroup, BLBC has significantly worse clinical outcomes with decreased recurrence-free survival and OS (13, 14).
BLBC and TNBC share many pathologic, molecular, and clinical features, resulting in a tendency to equate the two. However, these two groups are not strictly synonymous, with up to 10% to 20% of TNBC exhibiting a nonbasal genomic profile (9) and 15% to 40% of BLBC expressing either ER or HER2 (13, 15). The distinction between TNBC and BLBC may have prognostic and predictive implications, as studies have suggested that nonbasal TNBC may have a more favorable prognosis and demonstrate more sensitivity to chemotherapy (13,15,16).
The improved prognostic information provided by these molecular subgroups can aid in clinical decision making by more accurately identifying high-risk breast cancers. Issues regarding cost and reproducibility of gene expression profiling techniques currently limit its routine use. In an attempt to better delineate the basal-like cancers from the TNBC subgroup, a “five marker” method has been proposed combining the absence of ER, PR, and HER2 with the expression of either epidermal growth factor receptor (EGFR) or cytokeratin 5/6 (10). Although this method has demonstrated specificity for basal-like cancers, this definition has not been uniformly accepted because of concerns regarding sensitivity and reproducibility. In the absence of a consensus regarding the optimal method of defining the basal-like subgroup of patients, triple-negative status remains a practical surrogate.
Clinical Features
TNBC is associated with African American ethnicity, BRCA1 mutations, young age at diagnosis, lower socioeconomic status, and increased body weight (6,8,9,17–19). TNBC often presents aggressively as interval cancers in between screening mammography, and at diagnosis it is associated with a large tumor size and a high rate of lymph node positivity regardless of tumor size (6). TNBC is consistently associated with an increased risk of relapse and death, which persists after adjusting for other known prognostic factors (6,10,18,20,21). This risk of relapse remains significantly elevated even among those patients presenting with traditionally low-risk stage I (T1b/T1c N0) disease (22). The aggressive nature is further illustrated by the early peak of recurrent disease within 3 years of diagnosis and short survival following recurrence with most deaths occurring within 5 years of diagnosis (6,18,23,24). The pattern of recurrence for TNBC is also unique, with an increased propensity for lung and brain metastasis as the first sites of recurrence and a lower risk of bone metastasis (25–28).
TNBC and BRCA1
Up to 80% of BRCA1-associated breast cancers express a TNBC phenotype (29–32). In addition to triple-negative status, these tumors also share a number of clinicopathologic features, with TNBC including high-grade, genomic instability, p53 mutations, loss of X chromosome inactivation (33), central necrosis, and dense lymphocytic infiltrates (34–37). This overlap has lead to the hypothesis that nonmutational dysfunction of the BRCA1 pathway may be contributing to the molecular and clinical similarities between TNBC and BRCA1-associated breast cancers (38, 39). Although pre-clinical and clinical data support this hypothesis, the mechanism of BRCA1 dysfunction in sporadic TNBC has yet to be identified (40). This assumption is the basis for the development of some of the targeted therapies that are currently being investigated for TNBC.
An improved understanding of the role of BRCA1 in DNA repair has led to the development of therapy targeting DNA repair pathways. BRCA1 plays a key role in homologous recombination, one of two primary mechanisms of double-strand DNA repair (41). The homologous recombination pathway involves resynthesis of DNA from homologous sequences and results in a high-fidelity repair of double-strand DNA breaks. When BRCA1 expression is decreased, cells rely on alternative pathways to repair double-strand DNA breaks, such as nonhomologous end joining, a mechanism that allows for religation of DNA fragments without the use of a template. These alternative DNA repair mechanisms tend to be less efficient and more error prone than homologous recombination and translate into increased activation of apoptosis in response to DNA damage. On the basis of this understanding of the biology, one would predict that BRCA1-associated breast cancer and possibly TNBC would be more susceptible to agents that result in DNA damage, such as alkylators and platinum-based chemotherapy, as well as compounds that interfere with alternative DNA repair pathways.
 THE ROLE OF CHEMOTHER APY IN TNBC
THE ROLE OF CHEMOTHER APY IN TNBC
Chemotherapy remains the mainstay of treatment for TNBC. Numerous trials demonstrate efficacy of conventional cytotoxic therapy in the adjuvant, neoadjuvant (Table 1) (42–50), and metastatic setting. Although the majority of these trials were not designed to examine the benefit of chemotherapy exclusively in the TNBC population, retrospective subgroup analyses consistently report at least as much benefit of chemotherapy among patients with TNBC as other breast cancer subgroups.
Adjuvant Chemotherapy
The benefit of chemotherapy in ER-poor early breast cancer has been demonstrated in the Early Breast Cancer Trialists’ Collaborative Group 2005 meta-analysis (51). At 10 years, the use of polychemotherapy (non-taxane-based, typically about six cycles) versus no chemotherapy was associated with a significant reduction in relapse (45% vs 33%, hazard ratio [HR] 0.73, P <.00001) and death (33% vs 25%, HR 0.75, P = .0003) among patients with ER-negative early breast cancer. Although the impact of HER2 status on the analysis is not reported in this overview, on the basis of a retrospective subgroup analysis of several large randomized trials, patients with TNBC appear to derive at least as much benefit from chemotherapy as patients with HER2-positive breast cancer.
Neoadjuvant therapies for triple-negative breast cancer
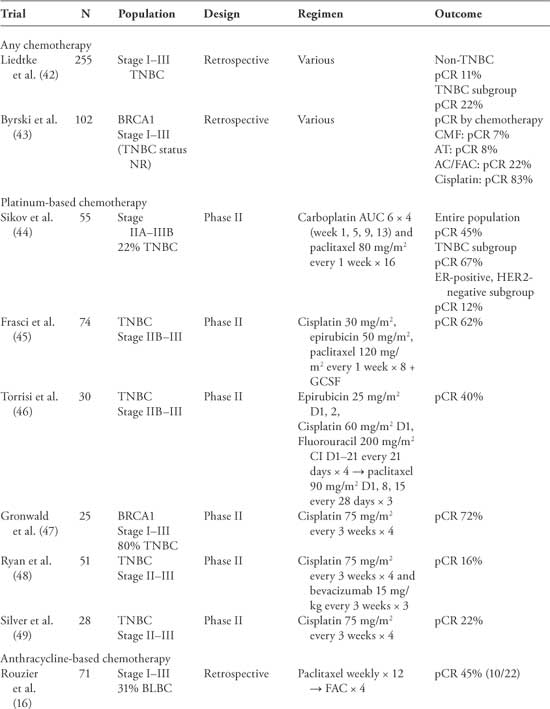

BLBC, basal-like breast cancer; CI, continuous infusion; GCSF, granulocyte colony-stimulating factor; NR, not reported; pCR, pathologic complete response; TNBC, triple-negative breast cancer.
Neoadjuvant Therapy
Both retrospective reviews and subgroup analysis of prospective neoadjuvant chemotherapy trials have provided important insights into the biology and chemosensitivity of TNBC compared with other subtypes of breast cancer. These studies have consistently reported increased objective response rates (ORR) and pathologic complete response rates (pCR) in TNBC compared with non-TNBC (Table 1) (16,21,42,52). Moreover, they have provided information regarding the relative sensitivities to specific chemotherapeutic agents and will be described elsewhere in this article.
Although patients with TNBC have an overall increased risk of relapse and death, those patients who do achieve a pCR with neoadjuvant treatment have a favorable prognosis that is not statistically different from non-TNBC (21,42,53,54). One of the largest studies to evaluate response to neoadjuvant therapy in TNBC compared with non-TNBC included 1,118 patients treated between 1985 and 2004 at the MD Anderson Cancer Center (42). Patients with TNBC who achieved a pCR had similar 3-year OS as the non-TNBC (94% and 98%, P = .24), whereas patients with TNBC who had residual disease after neoadjuvant therapy had a worse 3-year OS (68% vs 88%, P = .0001). Although there is no direct evidence to recommend the routine use of neoadjuvant therapy over adjuvant therapy, the neoadjuvant approach provides an opportunity to determine in vivo tumor responses to chemotherapy and remains an attractive option for the treatment of TNBC.
Dose-Dense or Metronomic Chemotherapy
In tumors with a high proliferative rate, such as TNBC, long intervals between the administration ofcytotoxic agents may allow for significant cancer cell repopulation and compromise the potential efficacy of these drugs. Dose-dense or dose-intense chemotherapy has been proposed as methods of optimizing delivery of cytotoxic agents with the goal of improving cancer outcomes. Two studies have examined the potential benefit of dose-dense chemotherapy in the TNBC population.
The CALGB 9741 trial was a 2 × 2 factorial design, randomizing 2005 lymph node-positive patients to doxorubicin, cyclophosphamide, and paclitaxel, either sequentially or in combination, and to either a conventional dosing (every 3 weeks) or dose-dense (every 2 weeks) schedule (55). In the study population as a whole, dose-dense chemo-therapy was associated with an improved DFS (HR 0.74, 95% confidence interval [CI] = 0.59–0.93; P = .01) and OS (HR 0.69, 95% CI = 0.51–0.93; P = .013). The estimated 4-year DFS rates for the dose-dense and conventional every 3-week schedules were 82% versus 75%, respectively. The 4-year OS was 92% in the dose-dense regimen and 90% for those receiving treatment every 3 weeks. In an unplanned subgroup analysis of the impact of ER status, it appears that the benefit of dose-dense therapy was driven predominantly by the ER-negative subgroup (DFS HR 0.71, 95% CI = 0.54–0.93, OS 0.68, 95% CI = 0.50–0.92) (56).
A study by the Gruppo Oncologico NordOvest group randomized 1,214 women to fluorouracil, epirubicin, and cyclophosphamide given either every 2 (dose dense) or 3 weeks (57). At a median follow-up of 10.4 years, there was no significant difference in outcomes based on dose density in the population as a whole (DFS HR 0.87, 95% CI = 0.67–1.13; P = .293; OS HR 0.88, 95% CI = 0.71–1.08; P = .21). The estimated actuarial 10-year survival was 80% in the dose-dense regimen and 78% in the every 3-week treatment (P = .35). A subgroup analysis by hormone status suggests a nonsignificant trend toward improved survival in the ER-negative population (DFS HR 0.78, 95% CI = 0.57–1.08; OS HR 0.80, 95% CI = 0.54–1.19). A recent pooled analysis of these two trials by Amir et al. reports a benefit of dose-dense chemotherapy, driven primarily by the additional benefit in the ER-negative subgroup (DFS HR 0.74, 95% CI = 0.60–0.90; P = .003; OS HR 0.73, 95% CI = 0.57–0.92; P = .008) (58).
Schedules of chemotherapy that benefit TNBC are not restricted to dose-dense administration, but may in fact be best exploited using a metronomic schedule. In a phase III neoadjuvant study of locally advanced breast cancer, a pCR of 43% was observed with weekly doxorubicin and daily oral cyclophosphamide as compared with a pCR of 26% with doxorubicin and intravenous cyclophosphamide given once every 3 weeks (59). Both arms received weekly paclitaxel. Taken together, the studies show the benefit of accelerated schedules (weekly or once every 2 weeks) of doxorubicin, cyclophosphamide, and paclitaxel compared with once every 3-week schedules in the adjuvant and neoadjuvant setting (60).
Metastatic Therapy
Patients with TNBC who present with meta-static disease do poorly with a median survival of 7 to 13 months following recurrence compared with greater than 20 months among non-TNBC patients (6, 7). Few trials have specifically examined the benefit of chemotherapy in the setting of metastatic TNBC. Despite the increased response rates to chemotherapy among TNBC noted in the preoperative setting, outcomes remain poor in the metastatic setting with the development of chemoresistance and rapid progression through subsequent lines of therapy (23).
 THE ROLE OF SPECIFIC CHEMOTHERAPY AGENTS IN TNBC
THE ROLE OF SPECIFIC CHEMOTHERAPY AGENTS IN TNBC
Anthracyclines
Anthracyclines have long been considered standard therapy for early breast cancer, with multiple trials demonstrating improvements in DFS and OS compared with non-anthracycline- containing regimens (61). Recent subgroup analyses of these trials have suggested that the benefit of anthracyclines may be restricted to the HER2-positive subgroup (62, 63), although the TNBC subgroup was not considered separately in these analyses. Anthracyclines in the neoadjuvant setting have activity in TNBC resulting in a pCR in the range of 8% to 36% (Table 1). A retrospective analysis of two multicenter phase III trials of anthracycline-based chemotherapy suggests that the TNBC subgroup derives at least as much benefit in terms of improvement in relapse-free survival (HR 0.35, 95% CI = 0.18–0.68) as the HER2-positive subgroup (HR 0.42, 95% CI = 0.17–1.05)(64). In contrast, a retrospective subgroup analysis of the MA.5 trial comparing cyclophosphamide, methotrexate, and fluorouracil (CMF) with cyclophosphamide, epirubicin, and fluorouracil (CEF) demonstrates improvement in 5-year OS favoring CMF in the core basal molecular subgroup (65). In addition, a recent preoperative study presented by Martin et al. suggests that patients with TNBC have a higher chance of residual disease at the time of surgery if they were treated with neoadjuvant doxorubicin compared with docetaxel (70% vs 32%, P = .01) (66). Whether this observation is due to a lack of sensitivity to anthracyclines among patients or an increased sensitivity to alkylating agents or taxanes among TNBC is unclear and will need to be confirmed in larger prospective trials.
Taxanes
Although there is some evidence to suggest that BRCA1-associated breast cancers may exhibit intrinsic taxane resistance, this observation may not translate to patients with sporadic TNBC. Retrospective subgroup analyses of several large prospective trials have consistently shown that patients with TNBC derive at least as much benefit from adjuvant taxane therapy as the population as a whole. The first trial that established the benefit of adding a taxane in TNBC was CALGB9344/ INT1048 (67). In this study, patients with TNBC or HER2-positive breast cancer derived the greatest benefit from the addition of four cycles of paclitaxel to four cycles of doxorubicin and cylophosphamide (AC).
In Eastern Cooperative Oncology Group (ECOG) 1199, 4,950 women were randomized to AC, followed by one of four taxane-containing arms, which consisted of paclitaxel or docetaxel given either weekly or every 3 weeks (68). Weekly paclitaxel was associated with improved DFS and OS compared with paclitaxel given every 3 weeks. This benefit was consistent for all HER2-negative tumors regardless of ER positivity.
In BCIRG 001 trial, 1,491 women with lymph node-positive breast cancer were randomized to six cycles of either FAC (fluorouracil, doxorubicin, cyclophosphamide) or TAC (docetaxel, doxorubicin, fluorouracil) in the adjuvant setting (69). In the whole trial population, TAC was associated with a significant improvement in both DFS and OS. In an attempt to identify predictive markers of response to taxanes, an unplanned subset analysis using immunohistochemistry was conducted using 1,350 (91%) patients who had pathology available for centralized review (70). In this subset review, TAC was associated with a trend toward improved 3-year DFS as compared with FAC among patients with TNBC (73.4% vs 60%, respectively; HR 0.50, 95% CI = 0.29–1.00; P = .051).
An increased relative benefit from the addition of taxane-based therapy to standard adjuvant polychemotherapy in TNBC has been suggested by several studies. The GEICAM 9906 study randomized 1,248 women with early breast cancer to either six cycles of FEC (fluorouracil, epirubicin, cyclophosphamide) or four cycles of FEC followed by weekly paclitaxel (FEC-P) for 8 weeks
(71). At 7-year follow-up, FEC-P was associated with superior DFS (75% vs 68%) and OS (84% vs 79%) compared with FEC. When the analysis was restricted to the TNBC subpopulation (n = 169) the improvement in DFS for FEC-P versus FEC was more pronounced (75% vs 56%, HR 0.58; P = .025). Likewise, in a multicenter trial reported by Loesch et al., 1,830 patients were randomized to either AC followed by paclitaxel every 3 weeks (AC-P) or doxorubicin and paclitaxel every 3 weeks followed by weekly paclitaxel (AP-weekly P)
(72). In the TNBC subgroup, 5-year DFS and OS were significantly better for those patients treated with AP-weekly P as compared with AC-P every 3 weeks (DFS 87% vs 79%, HR 0.59; P = .037).
The data regarding the potential incremental benefit of taxanes compared with anthracycline-based chemotherapy for the adjuvant treatment of TNBC are not conclusive, but if anything favors improved outcomes with the addition of a taxane to standard therapy (67–70,73).
Platinums
The use of DNA-damaging agents, such as platinum-based chemotherapy, is of particular interest in TNBC given the association between BRCA1-related breast cancers and TNBC. Preclinical studies of BRCA1-deficient cell lines have demonstrated exquisite sensitivity to platinum-based agents (74, 75). Furthermore, this sensitivity to platinum agents can be potentially reversed with the reintroduction of a functional BRCA1 gene, thus restoring DNA repair capacity (74, 75).
These preclinical studies are supported by neoadjuvant studies of BRCA1-associated breast cancers, which demonstrate pCR of more than 70% with platinum-based treatment (43, 47). In a registry analysis conducted by Byrski et al., 102 patients with breast cancer were identified with a BRCA1 mutation who received neoadjuvant chemotherapy. Twelve women received cisplatin-based treatment, and there was a pCR of 83%, whereas AC- or AT (doxorubicin and docetaxel)-treated patients had a pCR of 22% (n = 5/23) and 8% (n =2/25), respectively (43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 INTRODUCTION
INTRODUCTION