and Paul Telfer2
(1)
Department of Haematology, Guy’s and St Thomas’ Hospital, London, UK
(2)
Department of Haematology, Royal London Hospital, London, UK
Hydroxyurea
Hydroxyurea (or hydroxycarbamide), is currently the only licensed drug for long-term control of SCD.
Mechanism of Action
Hydroxyurea is a cytostatic agent which depletes the intracellular pool of deoxyribonucleotides required for synthesis and repair of DNA. This action is due to inhibition of the enzyme ribonucleotide reductase. The hematological effects include increases in fetal hemoglobin (HbF), mean cell volume (MCV), and steady state hemoglobin level. These are generally accompanied by a reduction in reticulocytes, white cells and platelets, and in markers of hemolysis including bilirubin and LDH. The reduction in intracellular sickling and in circulating dense red cells can be directly observed by comparing morphology of erythrocytes on the blood film before and during therapy (Fig. 18.1).
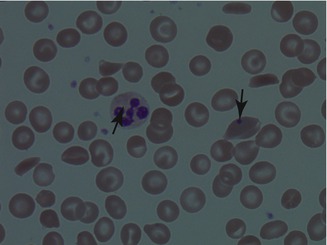

Figure 18.1
Blood film of patient with HbSS on hydroxyurea. Sickled cells are seen together with a red cell containing a Howell-Jolly body (down arrow). The changes associated with hydroxyurea therapy include macrocytosis (red cells are enlarged, in some cases almost to the size of the neutrophil), and neutrophil hypersegmentation (up arrow)
Augmentation of fetal hemoglobin is probably the principle mechanism of action, and this is thought to be caused by disruption of erythropoiesis with restriction of cell division in the marrow (stress erythropoiesis). Bone marrow suppression also results in a reduction in leukocyte count, which may be important since leukocytes are thought to play an important role in initiation of vaso-occlusion (See Chap. 1).The reduction in reticulocyte count and of sickled cells may also contribute to decreased red cell-endothelial interactions important in vaso-occlusion. Hydroxyurea also functions as a nitric oxide donor.
Efficacy
Prevention of Acute Complications
The Multicenter Study of Hydroxyurea (MSH) compared hydroxyurea with placebo in adults with HbSS and more than three acute painful crises per year (not necessarily requiring treatment in hospital). Hydroxyurea was commenced at 15 mg/kg and the dose escalated by 5 mg/kg/day at 12 weekly intervals aiming for maximum tolerated dose (MTD, see below). The trial was halted early, after a significant decrease in acute painful crises was confirmed. The final analysis showed a reduction in annual rate of acute painful crisis by about 50 %. There were also significant reductions in rates of acute chest syndrome and of crises requiring blood transfusion. These clinical effects were mirrored by an increase in HbF and MCV, which became apparent after 8 weeks of therapy and reached a peak at about 40 weeks.
Similar beneficial effects in acute crisis prevention have been shown in pediatric populations. Most recently the BABY-HUG study randomized children at 9–18 months of age to either hydroxyurea or placebo, irrespective of disease severity. The starting dose was 20 mg/kg/day and there was no dose escalation. This trial showed a significant decrease in pain episodes, dactylitis, acute chest syndrome, hospitalization and events requiring transfusion.
Prevention of Chronic Organ Damage
The BABY-HUG study was designed to test the concept that treatment started in infants would prevent or reduce chronic organ damage. The primary outcomes were measures of splenic and renal function (glomerular filtration rate) by radionucleotide scanning. There was no significant effect of hydroxyurea treatment on these measures. The SWiTCH trial examined the role of hydroxyurea in secondary prevention of stroke compared to transfusion. This trial is described in Chap. 7. The authors of the trial concluded that transfusion and chelation remain a better way of managing children with SCD, stroke, and iron overload. The TWiTCH trial is currently examining the role of hydroxyurea compared to transfusion in prevention of first stroke in children with abnormal Transcranial Doppler velocities, and results are awaited eagerly.
These unconvincing results in randomized controlled trials may relate to trial design and to short duration of follow-up. Improvements in organ function have been observed in prospective non-randomized studies. Examples include preservation of splenic function, secondary stroke prevention, lowering of Transcranial Doppler velocities, prevention of silent cerebral infarction, and treatment of pulmonary hypertension. For some of these, there are also studies that have failed to show a benefit of hydroxyurea. Some complications of SCD do not appear to be significantly improved whilst on hydroxyurea therapy, and may even deteriorate. We have noted this with avascular necrosis in childhood, priapism and lower limb ulceration.
The long-term effects of hydroxyurea on chronic organ damage are still not clarified and further randomized controlled studies will be important for refining the indications for therapy in both children and adults. These will be supplemented by long-term observational studies, such as The Long-Term Effects of Hydroxyurea Therapy in Children with Sickle Cell Disease (HUSTLE) which is a single center US study, gathering prospective data on end-organ damage and toxicity in children and planned to continue over at least 20 years.
Improved Life Expectancy
It is reasonable to expect that patients who have a good clinical and hematological response, and who can adhere to therapy in the long-term, will have improved life expectancy. This assumes that there are no unexpected adverse effects which are yet to emerge on long-term follow-up. The improvement could be related both to a reduction in life threatening acute crises, and a reduced risk of progressive organ damage.
So far, three non-randomized, long-term prospective studies provide some evidence supporting a reduction in mortality:
1.
Long-term follow-up of patients previously randomized in the MSH study has suggested an improvement in survival associated with the duration of hydroxyurea exposure.
2.
Retrospective data from a Brazilian pediatric cohort has shown improved survival in children treated with hydroxyurea for a median of 2 years with fewer deaths from acute chest syndrome and infections in the treated group.
3.
A prospective study from the Laikon Hospital in Athens, Greece in which severely affected adult patients (the majority with HbS β0 thalassemia) were treated with hydroxyurea and compared with less severely affected patients who were treated in the same center. There was improved survival at 10 years in patients on hydroxyurea compared to standard treatment (86 % compared to 64 %), as well as a decrease in acute painful crisis, acute chest syndrome and hospital admissions in the treated group.
On the other hand, there are studies in which hydroxyurea therapy does not appear to be a significant predictor of survival. Nevertheless, the evidence on reduced mortality may justify use of hydroxyurea in asymptomatic children and adults and for other indications where direct evidence for benefit does not yet exist (for example in patients with a tricuspid jet velocity >2.5 m/s on echocardiography). In order to be clearer on this important issue, more longitudinal follow-up and safety data is urgently required.
Adverse Effects
Although there were initial safety concerns, particularly in the pediatric population, long-term data has so far been reassuring. The HUG-KIDS study, an observational study of 5–16 year olds showed no adverse effects on growth and development. HUSOFT and its extension study, a prospective open-label trial in 6–28 month olds, unselected for disease severity showed very few toxicities. Long-term safety data in adults include the MSH follow-up report, and the Laikon Hospital data. There were no unexpected adverse events reported over 17 years of treatment in these studies. There are important on-going trials, including the HUSTLE study which will report on long-term toxicities in children. These need to be supplemented by prospective national or regional registry data, and we hope that the National Hemoglobinopathy Register will become a useful instrument for reporting on treatment, efficacy and adverse effects in the UK.
Myelosuppression
Myelosuppression is the most important short-term side effect and requires monitoring with regular blood tests. Although more common with higher doses, the incidence of myelosuppression is variable and can occur in patients who have been on hydroxyurea for many years, indeed there is some evidence that older patients may be more prone to developing myelosuppression at lower doses of hydroxyurea. This may be due to progressive infarction of active marrow and reduced bone marrow reserve in older patients. Trials which have used dose escalation to MTD have shown higher rates of myelosuppression than trials using a ‘minimal effective dose’ to achieve the therapeutic goal of control of acute painful crisis episodes.
Other Adverse Effects
Other short- to medium term adverse effects include nausea, which usually settles within a few weeks, hyperpigmentation of nails and hair thinning. These are sometimes unacceptable for young people.
Teratogenicity
In vitro hydroxyurea is mutagenic, and hydroxyurea treatment in laboratory animals can cause fetal abnormalities. In clinical experience, the majority of pregnancies where women have been exposed to hydroxyurea have resulted in normal babies. Women and men must be advised to use contraception whilst on hydroxyurea and to stop hydroxyurea at least 3 months before conceiving. If a woman becomes pregnant whilst on hydroxyurea, the drug should be stopped immediately and recommenced only after finishing breastfeeding. Hydroxyurea appears in breast milk at a concentration proportional to the oral dose, and is best avoided during breast feeding.
Effects on Spermatogensis
Sperm motility and sperm count are often decreased during hydroxyurea therapy and do not always return to normal, even several months after cessation. There are reports of long term azospermia in which hydroxyurea has been implicated, but in some cases these are more likely to be due to SCD. Testicular ischaemia and infarction has been suggested as a possible cause of reduced sperm count in men with SCD who have never been treated with hydroxyurea. An expert panel from the National Toxicology Program in the US has expressed concerns about the adverse effects of hydroxyurea on spermatogenesis based on data from young laboratory animals.
In summary, the long-term effects of hydroxyurea on spermatogenesis and male fertility are not yet clear, particularly in the case of boys who start medication at a young age. For post-pubertal males we recommend sperm count and if viable sperm are present, sperm cryopreservation before commencing hydroxyurea treatment. For parents of young children, this uncertainty needs to be communicated as part of the discussion of benefits and risks.
Risk of Malignancy
A potential increased risk of cancer with long-term hydroxyurea therapy has been one of the major reasons for reluctance of clinicians and patients to use hydroxyurea. The theoretical risk relates to its mechanism of action, in particular inhibition of the DNA repair process. There is some evidence from experience with myeloproliferative conditions (which are pre-malignant disorders of the blood seen mostly in adults) suggesting an increased risk of transformation to acute leukemia associated with hydroxyurea therapy, but this is largely explained by the preferential selection for treatment of more severe cases, which would be more likely to transform anyway.
At least two studies have examined for DNA changes in children taking hydroxyurea, including an ancillary of the BABYHUG study, which looked for chromatid breakages and aberrant recombination events in the immunoglobulin genes, and did not find any convincing in vitro evidence of genotoxicity.
In clinical practice, although there are case reports of malignant disease (particularly leukemias) in patients on hydroxyurea, the incidence does not appear to be at a higher frequency than in the general population. With up to 20 years of clinical experience since the first publication of the MSH study, and many thousands of patients from all over the world treated with hydroxyurea, the lack of reports indicating an increased risk of malignancy is reassuring.
Use in Clinical Practice
Indications
Table 18.1 lists suggested indications for hydroxyurea in children and adults.
Table 18.1
Suggested indications for hydroxyurea
Indication | Comments |
|---|---|
HbSS | |
>2 admissions to hospital per year with acute painful crisis | High-grade evidence, hydroxyurea should be recommended |
One or more episodes of acute chest syndrome | High-grade evidence, hydroxyurea should be recommended |
Recurrent painful episodes managed at home, but impacting on activity and quality of life | Moderate level evidence, hydroxyurea should be recommended |
Severe anemia and high hemolytic rate | Hydroxyurea likely to be of benefit and should considered |
HbSS with renal dysfunction or albuminuria | Moderate level evidence. Hydroxyurea should be considered in conjunction with ACEi or ARBs |
Chronic lung disease, pulmonary hypertension | Moderate level evidence Hydroxyurea should be considered in conjunction with specific treatment |
Recurrent priapism, leg ulceration, retinopathy, raised TR jet velocity | Low level evidence. Trial of hydroxyurea can be considered if risks outweigh the benefits |
Secondary stroke prophylaxis | Recommend hydroxyurea if patient is unable to receive blood transfusion |
Raised TCD velocity | Recommend hydroxyurea if patient is unable to receive blood transfusion |
Generally asymptomatic | Discuss the benefits and risks of hydroxyurea with patient to allow them to reach an informed decision |
HbSC and other genotypes | |
Features of severe disease (recurrent pain, acute chest syndrome) | Moderate level evidence. Hydroxyurea should be recommended |
Asymptomatic or mild disease | Inadequate evidence to currently recommend hydroxyurea treatment |
These indications are discussed in more detail elsewhere under the specific complications. The majority of trials in SCD have been limited to patients with HbSS or included small numbers of patients with other genotypes. A pragmatic approach for HbS β thalassemia and HbSC is to use the same indications as in patients with HbSS.
Patients and families need to be informed about hydroxyurea and its potential benefits, and the information repeated by clinic doctors and nurse specialists as opportunities present themselves, including at the annual review visit. We advise that a clinic-specific patient information sheet is made available to help in reaching a decision on therapy.
Dosing and Monitoring
The starting dose is usually 15 mg/kg. Maximum tolerated dose (MTD) will result in optimal HbF response, and better control of sickling, but at the expense of increased risk of side effects. MTD is reached by dose escalation of 5 mg/kg/day every 12 weeks, until neutrophil count falls below 1.5 × 109/l or platelet count below 80 × 109/l. At this point hydroxyurea is interrupted until blood counts recover. It is then restarted at a dose 2.5 mg/kg/day less. In some patients, the counts may already be reduced in pre-treatment steady state, due to causes such as splenomegaly or racial neutropenia. Here, the MTD is difficult to determine and patients should be monitored for a fall in blood counts below steady state. In general we would not recommend exceeding 35 mg/kg/day, the maximum dose used in the MSH study.
An alternative approach to dosing, which has been used in some European centers, is the minimum effective dose, which is determined pragmatically based on the presence or absence of painful crisis symptoms.
For monitoring, full blood count should be taken at 2 and 4 weeks and then monthly. More frequent monitoring should be done after each dose increase. Once on a stable dose, full blood should be checked every 8–10 weeks. Management of myelosuppression is covered in Table 18.2. A modest rise in Hb is often seen with hydroxyurea therapy but excessive rises are may result in hyperviscosity. An increase in Hb of >3 g/l to more than >120 g/l, should be considered an indication for dose reduction or venesection.
Table 18.2
Management of myelosuppression during hydroxyurea treatment
Neutrophils (×109/L) | Reticulocytes (×109/L) | Hemoglobin (g/l) | Platelets (×109/L) | Hydroxyurea |
|---|---|---|---|---|
≥1.5 × 109/L | >10 × 109/L | Within 30 g/l of baseline | ≥80 × 109/L | 100 % dose |
<1.5 × 109/L | <10 × 109/L | Fall of >30 g/l from baseline | <80 × 109/L | Stop treatment and recheck FBC until hematological recovery. Restart treatment using a dose reduced by 2.5 mg/kg (children) or 500 mg (adults). |
Barriers to Wider Prescription
It is reasonable to ask why hydroxyurea is not more widely used in managing SCD. It is the only disease-modifying drug currently licensed, and the evidence of its efficacy, at least in preventing acute vaso-occlusive crises, and improving hematological parameters, is convincing. Some clinicians suggest that there is sufficient evidence now to consider hydroxyurea for all children, even those who are asymptomatic, with the aim of preventing morbidity and mortality in the same way as we would control asthma with bronchodilators and anti-inflammatory drugs, or diabetes with insulin or hypoglycemic agents. Using words from a review document produced by the Agency for Healthcare Research and Quality in the US, “The risks of hydroxyurea are acceptable compared with the risks of untreated sickle cell disease”.
The approach in the UK and Europe so far has been more conservative, acknowledging these barriers to widespread use of hydroxyurea:
1.
Inadequate long-term data on efficacy in preventing chronic complications and improving life expectancy.
2.
Inadequate long-term data for reassurance about safety, in particular the risk of infertility in males.
3.
Perceptions and misperceptions about toxicities and lack of efficacy of hydroxyurea, sometimes based on anecdotal reports from individuals who have had a negative experience with hydroxyurea
4.
Inadequate health care resources for prescribing, monitoring and encouraging adherence.
Clinicians should discuss up-to-date evidence on efficacy and safety of hydroxyurea with their patients and provide written information to allow patients to make an informed choice about whether or not to take hydroxyurea. Sustained adherence to this drug is necessary to ensure clinical benefit, and this will only happen if the patient or parent is convinced that it is in the best interest of the patient to take it.
Blood Transfusion
Blood transfusion has an important role in both the acute and long-term management of SCD. In some acute situations (e.g. acute chest syndrome, acute multi-organ failure, acute anemic episodes in young children) transfusion can be lifesaving and should proceed with minimum delay. In general there have been very few prospective controlled trials to guide transfusion therapy in the acute setting, but several important studies have helped to define the role of chronic transfusion, particularly in primary and secondary prevention of cerebral ischemic damage. Although practice varies, the trend is for increasing use of transfusion, and large centers have seen a rapid increase in patients on planned regular transfusion over the past 10 years. Many patients have had significant clinical benefit from this approach, despite the inconvenience of regular visits to transfusion day care units. Transfusion is becoming better accepted partly because there are more options for control of long-term transfusional iron overload. There are important implications for health care resources as this practice is expanded, and further data on benefits, risks and quality of life are required.
Objective of Transfusion
Although the objectives of transfusion differ in the settings of an acute sickle crisis and long-term primary or secondary prevention, the expected benefits include:
Correction of anemia
Improving oxygen carrying capacity of the blood
Control or reversal of an acute sickling complication
Control or prevention of chronic vascular or tissue damage
Beneficial Effects of Transfusion
These include:
Improvement of blood rheology by ‘diluting’ sickle hemoglobin containing red cells. This effect needs to be balanced against increased blood viscosity if hemoglobin level is too high. The optimal hemoglobin level is about 110 g/l
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree