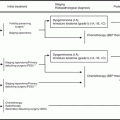
Fig. 14.1
Treatment strategy of recurrent epithelial ovarian cancer
Table 14.1
Important clinical trials for recurrent ovarian cancer
Study (y) | Drug | n | Primary endpoint | RR(%) | PFS(M) | OS(M) | Comments |
Platinum-sensitive disease | |||||||
Parmar MK et al. (2003)[7] | Platinum (71%: Carboplatin, 17%: CAP) | 410 | OS | 54 | 13 | 29 | OS was significant (HR 0.82; 95% CI 0.69-0.97; p = 0.02). PFS was significant (HR 0.76; 95% CI 0.66-0.89; p = 0.0004) |
ICON4/OVAR2.2 | Platinum+Taxane (80%: TC, 10%: TP) | 392 | 66 | 10 | 24 | ||
Pfisterer J et al. (2005)[8] | Carboplatin | 178 | PFS | 47 | 8.6 | PFS was significant (HR 0.72; 95% CI 0.58-0.90; p = 0.0032). HR for OS was 0.96 (95% CI 0.75-1.23; p = 0.7349). | |
OVAR2.5 | Carboplatin+Gemcitabine | 178 | 31 | 5.8 | |||
Pujade-Lauraine E et al. (2010)[16] | Carboplatin + Pegylated liposomal doxorubicin | 466 | PFS | 21 | 11.3 | 30.7 | Test for noninferiority of CD yielding p < 0.001. |
CALYPSO trial | Pegylated liposomal doxorubicin+Paclitaxel | 501 | 20 | 9.4 | 33 | ||
Monk BJ et al. (2010)(2012)[20] | Pegylated liposomal doxorubicin | 335 | PFS | 18.8 | 5.8 | 18.9 | The trial included 35% platinum-resistant disease. Platinum-sensitive PFS; 9.2M vs7.5M (HR 0.73; 95% CI 0.56-0.95; p = .0170) |
Pegylated liposomal doxorubicin+Trabectedin | 337 | 27.6 | 7.3 | 22.2 | |||
Carboplatin+Gemcitabine | 233 | PFS | 57.4 | 8.4 | 32.9 | PFS was significant (HR, 0.484; 95% CI 0.388-0.605; p < 0.0001). OS was insignificant (HR 0.95; p = 0.65) | |
OCEANS trial | Carboplatin+Gemcitabine+Bevacizumab | 247 | 78.5 | 12.4 | 33.6 | ||
Ledermann et al. (2012)[34] | Platinum based chemotherapy | 129 | PFS | 4.8 | 27.8 | Both PFS and OS were significant. In cohort of BRCA mutation was significant (OS:34.9 vs 30.2 months; HR 0·62; p = 0.025). In cohort of BRCA wild-type was insignificant (OS:24.5 months with olaparib vs 26.6 months with placebo; HR 0.83 ; p = 0.37). | |
Platinum based chemotherapy+ maintenance olaparib | 136 | 8.4 | 29.8 | ||||
Liu et al. (2014)[38] | Olaparib | 46 | PFS | 9 | PFS was significant (HR 0.42; 95% CI 0·23-0·76; p = 0·005). | ||
Olaparib+Cediranib | 44 | 17.7 | |||||
Oza A et al. (2015)[28] | Carboplatin+Paclitaxel | 75 | PFS | 9.6 | PFS was significant (HR 0.51; p = 0.0012), and also significantly improved PFS in BRCA-mutated patients(HR 0.21; p = 0.0015). Final OS did not differ between each arm even by the subset analysis only for BRCA mutated patients. | ||
Carboplatin+Paclitaxel+maintenance olaparib | 81 | 12.2 | |||||
Ledermann J et al. (2016)[81] | Platinum based chemotherapy | 118 | PFS | 8.7 | |||
ICON6 | Platinum based chemotherapy+ cediranib | 174 | 9.9 | PFS of maintenance cediranib was more significant than only plainum based chemotherapy (HR 0.56, 95% CI, 0.44–0.72, p < 0·0001). | |||
Platinum based chemotherapy+ cediranib+maintenance cediranib | 164 | 11 | |||||
Mirza MR et al. (2016)[40] | Platinum based chemotherapy | gBRCA: 65 | PFS | 5.5 | The niraparib group had a significantly longer PFS than the placebo group, regardless of presence (gBRCA cohort, median PFS, 21.0 vs 5.5 months; HR, 0.27) or absence (non-gBRCA without HRD cohort, median PFS, 9.3 vs 3.9 months; HR, 0.45) of gBRCA mutation or HRD status | ||
ENGOT-ov16/NOVA | non-gBRCA:116 | 3.9 | |||||
Platinum based chemotherapy+niraparib | gBRCA: 138 | 21 | |||||
non-gBRCA:234 | 9.3 | ||||||
Platinum-resistant disease | |||||||
Naumann RW et al. (2013) | Pegylated liposomal doxorubicin | 31 | PFS | 15 | 12 | PFS was significant (HR 0.63; 95% CI, 0.41-0.96 ; p = 0.031). | |
PRECEDENT trial | Pegylated liposomal doxorubicin+vintafolide | 60 | 17 | 22 | |||
Weekly-paclitaxel | 458 | PFS | 30 | 5.4 | 17.3 | PFS was significant (HR 0.66; 95% CI 0·57–0·77; p < 0·0001). OS was insignificant | |
TRINOVA 1 trial | Weekly-paclitaxel+trebananib | 461 | 38 | 7.2 | 19 | ||
Pegylated liposomal doxorubicin/topotecan/Weekly-paclitaxel | 182 | PFS | 12 | 3.4 | 13.3 | PFS was significant (HR 0.484; p < 0.001). Overall RR; p = 0.001. OS was Insignificant (HR 0.85). | |
AURELIA trial | Pegylated liposomal doxorubicin/topotecan/Weekly-paclitaxel+bevacizumab | 179 | 31 | 6.7 | 16.6 | ||
14.2 Platinum-Sensitive Recurrent Ovarian Cancer
14.2.1 Chemotherapy for Platinum-Sensitive Disease
Several reports are proving the significance of secondary debulking surgery (SDS) in patients with sensitive recurrence (≥6 months) [11, 12], and the National Comprehensive Cancer Network (NCCN) guidelines of ovarian cancer [13] provide the possible benefit of SDS for the patient before considering the selection of chemotherapeutic agent. Platinum-based combination chemotherapy is superior in the patients with recurrence ≥6 months after first-line chemotherapy, and combination therapy including a platinum agent is strongly recommended in guidelines [7, 13–15]. Currently, the most commonly recommended regimens for platinum-sensitive patients are paclitaxel plus carboplatin (TC), gemcitabine plus carboplatin (GC), and pegylated liposomal doxorubicin plus carboplatin (PLD-C) [7, 8, 14, 16].
A phase III trial of ICON4 and AGO-OVAR-2.2 proved paclitaxel plus platinum agent improved OS (hazard ratio (HR) 0.82; 95% CI 0.69–0.97, p = 0.02) and PFS (HR 0.76, p = 0.0004) better than conventional platinum-based therapy with a median follow-up of 42 months. The 2-year OS rate of the paclitaxel group significantly improved 7% over the conventional treatment groups (57% vs. 50%), and median survival increased an average of 5 months (29 vs. 24 months). The 1-year PFS rate also improved with a significance of 10% (50% vs. 40%) and a median PFS increase of 3 months (13 vs. 10 months) in the paclitaxel regimen [7]. A comparison between the administration of TC versus carboplatin in platinum-sensitive ROC patients showed a significant difference in the RR in favor of the TC group (75.6% vs. 50%) of the phase II trial in 78 patients [14]. Although mucositis, alopecia, myalgia/arthralgia, and peripheral neuropathy were more frequent in the paclitaxel-containing group, no significant differences were observed in grades 3–4 hematological toxicity. The median time to progression was superior in the combination group (49.1 vs. 33.7 weeks, p = 0.021).
Another phase III randomized controlled trial (RCT) study by Gynecologic Cancer InterGroup (GCIG) compared the use of carboplatin alone versus GC in platinum-sensitive ROC [8]. With a median follow-up of 17 months, GC treatment significantly improves the median PFS (8.6 vs. 5.8 months; HR 0.72, p = 0.0032) of patients. The RR for the GC was higher than carboplatin (47.2% vs. 30.9%, p = 0.0016), and patients treated with GC showed significant improvement in symptomatic participants such as abdominal pain. OS was not precisely analyzed due to a lack of statistical power.
A large number of 975 patients joined the phase III RCT of CARIPSO [16, 17], the purpose of which was the evaluation for the efficacy and safety of the combination of PLD with carboplatin (CD) compared with standard TC against platinum-sensitive ROC. PFS for the CD was statistically superior to the TC arm (HR, 0.821; p = 0.005); median PFS was 11.3 versus 9.4 months, respectively. However, in the final analysis of OS, median survival times were 30.7 months in the CD and 33.0 months in the TC (HR, 0.99; p = 0.005). Non-prolonged OS probably originated in the fact that 90% of patients in either arm of CALYPSO underwent post-study therapy, and a majority (68%) of the patients with TC received a crossover of PLD as post-study therapy. The significant influence factors for OS include TFI ≥ 12 months, ECOG PS 0, CA125 < 100Uml, nonmeasurable disease, and one disease site.
CD was reassessed in Japanese patients during a phase II trial in platinum-sensitive ROC [18]. Although the most frequent grades 3–4 toxicities were neutropenia (82%), thrombocytopenia (51%), and anemia (17%), severe non-hematological toxicities were not found. The efficacy of CD reported that 82% of the patients with evaluable CA-125 achieved ≥50% reduction compared with pretreatment results. The overall RR was 52%, and the survival outcomes were almost even (the median PFS and OS rates were 10.7 and 38.8 months) compared to the previous study [16–18].
Adverse effects in both combination regimens were reported. CD was associated with palmar-plantar erythrodysesthesia (PPE) (12 vs. 2.2%) and grades 3–4 thrombocytopenia (15.9 vs. 6.2%). TC was associated with consistent toxicities including alopecia (83.6 vs. 7%), grade 2 or higher neuropathy (26.9 vs. 4.9%), and neutropenia (45.7 vs. 35.2%). Hypersensitivity reaction (HSR) was a serious complication for taxane- and platinum-accumulative patients. A higher percentage of associated ≥ grade 2 HSR occurred in the TC (18.8%) or only carboplatin (23%) groups, whereas CD-induced HSR was only 0–5.6%. Although the mechanism is still unclear, the additional PLD likely reduced HSR [16, 19].
Regarding the non-platinum agent effective for platinum-sensitive ROC, a marine-derived antineoplastic agent of trabectedin was compellingly proved by an OVA-301 phase III RCT under the comparison between trabectedin plus PLD and PLD alone against ROC. This study revealed that median PFS was superior in the group containing trabectedin (7.3 vs. 5.8 months, HR, 0.79; 95% CI, 0.65 to 0.96; p = 0.019), although OS was not significant (p = 0.151). This study included platinum-resistant (PFI < 6 months)/platinum-sensitive (PFI ≥ 6 months) recurrences. Notably, subsequent analysis of partially sensitive recurrence (PFI from 6 to 12 months) OS in addition to PFS significantly favored the trabectedin plus PLD combination (HR 0.65; 95% CI, 0.45–0.92; p = 0.0152) [20, 21].
14.2.2 Targeted Therapies for Platinum-Sensitive Disease
14.2.2.1 Antiangiogenic Agents for Platinum-Sensitive Disease
An angiogenic inhibitor of bevacizumab is a monoclonal antibody against vascular endothelial growth factor (VEGF) and inhibits activity of human VEGF to reduce neovascularization, inhibit tumor growth, and decrease ascite formation. Bevacizumab is the molecular targeted agent, which was initially well proven to have a therapeutic effect for both primary and recurrent ovarian cancers.
Regarding the administration of bevacizumab in ROC, a phase II trial GOG 170 including 41% of platinum-sensitive disease firstly reported the efficacy and tolerability of single-agent bevacizumab. RR was 21%; an additional 52% was reported as stable disease, and 40% were progression-free for a duration of at least 6 months. Platinum sensitivity and prior chemotherapy regimens were not significant influences for PFS under the intravenous bevacizumab therapy [22, 23].
OCEANS is a phase III RCT comparing gemcitabine plus carboplatin with or without bevacizumab in 484 patients of platinum-sensitive ROC. Eligible criteria included no prior chemotherapy in the recurrent stage, no existing measurable disease, and no prior treatment by VEGF pathway-targeted therapy including bevacizumab. The addition of bevacizumab led to a significant increase in PFS compared with placebo (median PFS, 8.4 vs. 12.4 months; p < 0.0001). There was also a higher objective RR (78.5% vs. 57.4%; p < 0.001) and prolonged duration of response (10.4 vs. 7.4 months; HR = 0.534). GI perforations did not occur during the treatment, but two patients suffered GI perforation after the study treatment, both on the 69th day after the last administration. The final OS was comparable between both arms (GC plus bevacizumab 33.6 months; GC plus placebo 32.9 months; HR = 0.95; p = 0.65). Thirty-eight percent of patients in the GC plus placebo arm received subsequent bevacizumab after the trial, making the data difficult to determine a significant improvement in OS [24, 25].
MITO-16/MaNGO OV-2 is an ongoing study to evaluate the efficacy of bevacizumab administered in both first- and second-line therapies. The protocol includes PLD-C or GC or TC with/without bevacizumab as the second-line therapy to platinum-sensitive ROC treated by the first-line therapy including bevacizumab (beyond progression disease (PD)).
14.2.2.2 PARP Inhibitor for Platinum-Sensitive Disease
Olaparib is an oral poly(ADP-ribose) polymerase inhibitor (PARPi), which has successively proven antitumor activity in BRCA1−/BRCA2-mutated cancers, including ROC patients [26–33]. In evaluation of olaparib on tumor response across a spectrum of malignancies in BRCA1−/BRCA2-mutated ROC, RR were 31.1%, and stable disease >8 weeks was 40% even in platinum-resistant cases [29]. The Food and Drug Administration (FDA) in the USA approved olaparib for the fourth-line treatment of BRCA-deficient ovarian cancer. It was permitted based on the efficacy of olaparib monotherapy on 265 patients with platinum-sensitive HGSOC receiving more than two prior regimens of chemotherapy [26, 27, 34]. Almost all patients were examined BRCA status, of whom about half (olaparib, 56%; placebo, 50%) had BRCA mutation. The BRCA-mutated olaparib maintenance cohort significantly improved PFS over placebo (median 11.2 vs. 4.3 months; HR 0.18; p < 0.0001); BRCA wild type also showed advantages in PFS compared to placebo (median 7.4 vs. 5.5 months; HR 0.54; p = 0.0075). Updated analysis revealed a significant OS advantage in the olaparib cohort of BRCA mutation (median OS, 34.9 vs. 30.2 months; HR, 0.62; p = 0.025), although the BRCA wild type in the olaparib group showed no significant changes (median OS, 24.5 vs. 26.6 months; HR, 0.83; p = 0.37). Following this retrospective review, a prospective RCT phase II trial was examined [34]. In all patients, PFS was significantly longer in olaparib arm than placebo group (median, 8.4 vs. 4.8 months; p < 0.001). OS advantage also appeared in maintenance of olaparib (median, 29·8 vs. 27·8 months) and in the cohort of BRCA mutation (34.9 vs. 30.2 months; HR 0·62; p = 0·025). However, OS in patients with BRCA wild type were insignificant (24.5 months with olaparib vs. 26.6 months with placebo; HR 0·83; p = 0.37) [27]. Regarding the efficacy of olaparib combined with cytotoxic agents, a phase II trial was performed for platinum-sensitive HGSOC and evaluated oral olaparib plus TC, followed by olaparib as a maintenance therapy [28]. Patients in olaparib plus TC arm had a lower risk of disease progression than the only chemotherapy arm (median PFS, 12.2 vs. 9.6 months; HR 0.51; p = 0.0012) and also significantly improved PFS in BRCA-mutated patients (HR 0.21; p = 0.0015). Final OS did not differ between each arm even by the subset analysis only for BRCA-mutated patients. At least 10% higher adverse events in the olaparib arm than in the placebo arm were alopecia, nausea, neutropenia, diarrhea, headache, peripheral neuropathy, and dyspepsia. Although 19% of patients had adverse events leading to treatment discontinuation, no fatal adverse events occurred.
Other PARPi drugs include rucaparib and veliparib and have been examined in phase II trials for ROC [35–37]. Various clinical trials of PARPi for ROC are being investigated. Both SOLO-2 (NCT01874353) and ENGOT-ov16/NOVA (NCT01847274) are phase III RCT comparing olaparib (SOLO-2)/niraparib (NOVA) versus placebo in the patients with BRCA mutation, two prior treatments of platinum-based chemotherapy and platinum-sensitive recurrent HGSOC. SOLO-3 (NCT02282020) is comparing olaparib monotherapy versus single-agent chemotherapy in platinum-sensitive ROC. These results have the possibility to alter the standard care for ROC, including the FDA-approved usage of olaparib. The combination of olaparib and cediranib showed survival advantage (PFS, 17.7 vs. 9 months; HR, 0.42; p = 0.005) compared to monotherapy, even in either BRCA-unknown status or BRCA wild-type patients (ORR, 76 vs. 32%, p = 0.006; PFS, 16.5 vs. 5.7 months; p = 0.008) [38]. This combination is now being assessed in two phase III trials for platinum-sensitive ROC with germline BRCA mutation (NCT02446600) and platinum-resistant ROC with no prior antiangiogenic agents (NCT02502266).
Rucaparib is another promising oral PARP1&2i that was proved by a phase II ARIEL trial (NCT01891344) in patients with platinum-sensitive recurrent HGSOC. The trial investigated not only RR but also molecular homologous recombination deficiency (HRD) signature to detect patients with clinical benefit to PARPi. Based on the defined molecular signature by ARIEL2, phase III ARIEL3 trial (NCT1968213) in platinum-sensitive disease stratified three groups before randomization and assessed the rucaparib maintenance compared to placebo.
Niraparib is also PARP1&2i and underwent a phase I study [39], which decided that 300 mg/day is the tolerable dose and achieved 40% RR in HGSOC. Although there is no completed phase II study at present, some phase I–II studies are ongoing (NCT02354586, NCT02354131, NCT02657889). The aforementioned phase III RCT of ENGOT-ov16/NOVA (NCT01847274) evaluated niraparib versus placebo by 553 patients with platinum-sensitive ROC. The niraparib group had a significantly longer PFS than the placebo group, regardless of presence (gBRCA cohort, median PFS, 21.0 vs. 5.5 months; HR, 0.27) or absence (non-gBRCA without HRD cohort, median PFS, 9.3 vs. 3.9 months; HR, 0.45) of gBRCA mutation or HRD status. Interestingly, PFS was also longer in the non-gBRCA cohort with HRD than the placebo group (non-gBRCA with HRD cohort, median PFS, 12.9 vs. 3.8 months; HR, 0.38; 95% CI, 0.24 to 0.5). The reported toxicities of grade 3 or 4 were thrombocytopenia (33.8%), anemia (25.3%), and neutropenia (19.6%) [40].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree