Locally advanced non–small cell lung cancer is a heterogeneous disease with typically poor outcomes. Select patients may benefit from the integration of surgery, whereas patients with bulky, multistation, or contralateral (N3) mediastinal involvement are managed with definitive chemoradiation. Attempts to improve outcomes through induction, consolidation, or maintenance chemotherapy or radiation dose escalation have not demonstrated a survival benefit. Current research efforts focus on the integration of novel systemic agents that exploit tumor-specific driver mutations, augment antitumor immune response, or enhance radiation sensitivity.
Key points
- •
Stage III non–small cell lung cancer (NSCLC) is a heterogeneous disease.
- •
Concurrent chemoradiation with platinum/etoposide and carboplatin/paclitaxel is the standard of care for unresectable disease.
- •
60 Gy in standard fractionation remains the standard of care for radiation dose.
- •
Integration of novel immunotherapeutic and molecular targeted therapies is a promising area of investigation.
Introduction
Stage III NSCLC comprises the most heterogeneous group of patients and accounts for one-third of all patients diagnosed with lung cancer. Despite this heterogeneity, chemoradiation is the treatment of choice for the majority of patients. The 2-year and 5-year overall survival (OS) rates are estimated at 55% and 36%, respectively, for patients with stage IIIA disease and 34% and 19%, respectively, for patients with stage IIIB disease.
Introduction
Stage III NSCLC comprises the most heterogeneous group of patients and accounts for one-third of all patients diagnosed with lung cancer. Despite this heterogeneity, chemoradiation is the treatment of choice for the majority of patients. The 2-year and 5-year overall survival (OS) rates are estimated at 55% and 36%, respectively, for patients with stage IIIA disease and 34% and 19%, respectively, for patients with stage IIIB disease.
Patient evaluation
To accurately classify a patient within this diverse stage, a comprehensive work-up is imperative. After a thorough history and physical examination, staging focuses on the pathologic and radiographic assessment of primary and/or nodal disease and assessment of a patient’s physiologic reserve and expected tolerance to planned therapies.
Initial imaging includes a computerized tomography (CT) of the chest to delineate local and regional disease and anatomic relationship to normal thoracic structures, whole-body positron emission tomography (PET)/CT for regional and distant staging, and a brain magnetic resonance imaging (MRI) to evaluate for intracranial metastases. Pathologic disease confirmation should be obtained from the most accessible tumor site, whether primary or nodal. Primary tumors may be accessed by CT-guided fine-needle aspiration or core biopsy, surgically via video-assisted thoracoscopic surgery, or by endobronchial ultrasound–guided fine-needle aspiration for centrally located tumors adjacent to bronchus. Nodal deposits may be accessed via endobronchial ultrasound (levels 2R/2L, 4R/4L, 7, and 10R/10L), esophageal ultrasound (levels 5, 7, 8, and 9), mediastinoscopy, mediastinotomy, or video-assisted thoracoscopic surgery.
If surgical management is being considered, comprehensive pathologic mediastinal staging is recommended ( Fig. 1 ) especially because the rates of both false-positive and false-negative PET/CT interpretations for mediastinal nodes remain high. A meta-analysis of 28 studies, including 3255 patients, identified sensitivity and specificity of 0.67 and 0.87, respectively, for PET/CT in the nodal staging of NSCLC. Patients with bulky, multistation mediastinal adenopathy less commonly undergo comprehensive pathologic nodal staging and are managed nonsurgically. Biopsy of radiographically borderline nodes in nonoperative patients, however, may also have an impact on radiation therapy target delineation for definitive chemoradiation.
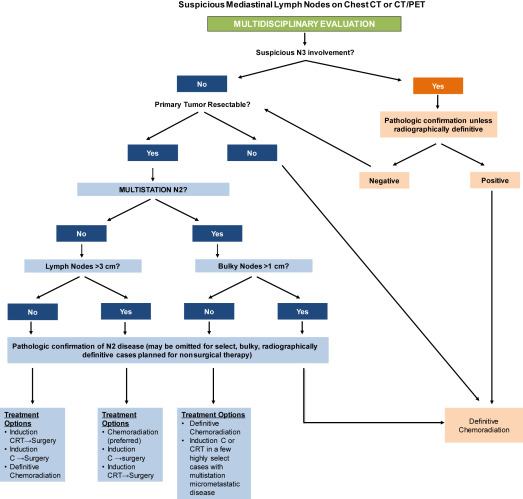
For patients under consideration for surgical resection, assessment of performance status, pulmonary reserve, and comorbidities is crucial. Pulmonary function tests with spirometry and diffusion capacity are a standard component of a presurgical work-up and are a helpful baseline prior to nonsurgical therapy. Threshold values for resectability vary among surgeons, but an estimated postoperative forced expiratory volume in the first second of expiration or diffusing capacity of the lungs for carbon monoxide of less than 30% indicates an increased risk for complications after resection. Low-technology exercise tests, including stair climbing and shuttle walk, as well as cardiopulmonary exercise tests, are also used to determine expected operative risk.
Resectable stage III non–small cell lung cancer
The role of surgery in the treatment of stage III NSCLC remains controversial. A small body of evidence suggests that a subset of patients with pathologic N2 disease may benefit from surgery after induction chemoradiotherapy or chemotherapy. The most persuasive data come from a subset analysis of the North America Intergroup trial (INT0139; Radiation Therapy Oncology Group [RTOG] 9039) ; 429 patients were randomized to receive 2 cycles of cisplatin/etoposide (PE) concurrently with 45 Gy radiation followed by surgery or continued radiation to 61 Gy. There was no survival difference between the 2 arms but a subset analysis by the extent of surgery showed a significant survival advantage for patients undergoing lobectomy, with a median survival (MS) of 34 months after lobectomy versus 22 months for the nonsurgical arm ( P = .002) and a 5-year OS of 36% versus 18%, respectively. Patients undergoing pneumonectomy had a nonsignificant but numerically worse outcome, with an MS of 19 months with surgery compared with 29 months without surgery and 5-year OS rates of 22% versus 24%, respectively. A higher than expected perioperative mortality in the pneumonectomy arm of 26% contributed to these results. In addition to the extent of surgical resection, retrospective analyses show that the number and size of involved nodes and nodal response to induction are important factors. Lymph node(s) greater than or equal to 1 cm on CT (clinical N2 disease), multistation involvement, or nodes greater than 3 cm portend survival decrements, and mediastinal tumor clearance with induction therapy is associated with prolonged survival. In INT0139, patients who cleared their mediastinal disease (N0) had an MS of 34.4 months compared with 26.4 months for patients with N1–N3 or unknown N status. Based on these data, it is recommended that patients undergo repeat pathologic evaluation of the mediastinum prior to definitive surgery; if disease is found, the resection should be aborted and the patient should receive or complete definitive chemoradiation.
The optimal induction regimen is unknown. A randomized phase III trial conducted by the Swiss Group for Clinical Cancer Research evaluated induction docetaxel and cisplatin versus docetaxel plus cisplatin followed by radiotherapy in resectable pathologically proved stage III N2 disease. There was no difference in event-free survival between the arms, suggesting chemotherapy alone was sufficient prior to resection. The trial had several limitations, however, including its small sample size, 11 years of accrual, sequential radiotherapy design, and lack of an OS endpoint. Several attempts to conduct randomized trials comparing the 2 approaches have failed due to poor accrual. Current guidelines allow for chemotherapy alone or chemoradiation as the induction regimen. For patients treated without neoadjuvant radiotherapy, adjuvant postoperative radiotherapy (PORT) may be considered after surgical management of N2 disease. A large meta-analysis, including 2128 patients from 9 randomized trials, identified a survival decrement to the use of PORT for N0–N1 patients with no apparent survival impact for N2 disease, although many of the analyzed trials used outdated radiation techniques, including cobalt. Subsequent population-based studies using modern radiation techniques have suggested a small OS benefit to the use of PORT for N2 disease. It is anticipated that the currently accruing Lung Adjuvant Radiotherapy Trial trial in Europe, in which resected N2 patients are randomized between PORT and no PORT, should provide a definitive answer to this question. All patients should be discussed at a multidisciplinary tumor board and a tailored treatment plan devised.
Unresectable stage III non–small cell lung cancer
A majority of patients with stage III disease are unresectable. Radiation as monotherapy cures fewer than 10% of patients. Multiple studies show that patients with unresectable disease may achieve long-term survival when radiation therapy is combined with chemotherapy ( Table 1 ). The landmark study performed by Dillman and colleagues demonstrated a 4-month improvement in MS and a doubling of long-term survivors after induction chemotherapy with cisplatin and vinblastine followed by thoracic radiation compared with radiation therapy alone. RTOG and the Eastern Cooperative Oncology Group conducted a confirmatory trial that favored the combination arm. The results were also corroborated by a French multicenter randomized study. Based on the positive results from these 3 trials, the addition of chemotherapy to radiotherapy became the standard of care for the management of locally advanced NSCLC.
| Author, Reference | N | Treatment | Median Survival (mo) | 2 y Overall Survival (%) | 5 y Overall Survival (%) | P Value |
|---|---|---|---|---|---|---|
| Dillman et al, 1990 | 78 | Cisplatin-vinblastine + radiation therapy | 13.8 | 26 | 19 | P = .0066 |
| 77 | RT alone | 9.7 | 13 | 7 | ||
| Sause et al, 2000 | 149 | Cisplatin-vinblastine + radiation therapy | 13.2 | 32 | 8 | P = .04 |
| 152 | RT alone | 11.4 | 21 | 5 | ||
| Le Chevalier et al, 1994 | 176 | Cisplatin-vindesine-cyclophosphamide-lomustine + radiation therapy | 12 | 21 | 11 a | P = .08 |
| 177 | Radiation therapy alone | 10 | 14 | 5 a |
Timing of Chemotherapy and Radiotherapy
The next set of studies investigated timing of chemotherapy and radiation ( Table 2 ). The West Japan Thoracic Oncology Group was the first to demonstrate that concurrent compared with sequential chemoradiation significantly improved response rate and survival. Confirmatory trials performed by cooperative groups in France, the Czech Republic, and the United States (RTOG 9410), also showed a survival benefit for the concurrent approach. A meta-analysis of concurrent versus sequential chemoradiation data from 6 randomized trials involving 1205 patients with median follow-up of 6 years demonstrated a significant survival benefit for concurrent chemoradiation (hazard ratio 0.84; 95% CI, 0.74–0.95; P = .004), with an absolute benefit of 5.7% at 3 years and 4.5% at 5 years. Based on these results, concurrent therapy is considered standard for good-performance status patients. Sequential chemoradiation remains an option for patients with a marginal performance status, and poor-performance patients are typically treated with radiation alone.
| Author, Reference | N | Treatment | OR (%) | Median Survival (mo) | 2 y Overall Survival (%) | 5 y Overall Survival (%) | P Value |
|---|---|---|---|---|---|---|---|
| Furuse et al, 1999 | 156 | Concurrent | 84 | 16.5 | 34.6 | 15.8 | P = .03998 |
| 158 | Sequential | 66.4 | 13.3 | 27.4 | 8.9 | ||
| Fournel et al, 2005 | 100 | Concurrent | 49 | 16.3 | 39 | 21 b | P = .24 |
| 101 | Sequential | 54 | 14.5 | 26 | 14 b | ||
| Zatloukal et al, 2004 | 52 | Concurrent | 80 | 16.6 | 34.2 | 18.6 a | P = .023 |
| 50 | Sequential | 47 | 12.9 | 14.3 | 9.5 a | ||
| Curran et al, 2011 | 193 | Concurrent | 70 | 17 | — | 16 | P = .46 |
| 195 | Sequential | 61 | 14.6 | — | 10 |
Selection of Chemotherapy Regimen
All cytotoxic chemotherapy agents used to treat metastatic lung cancer exhibit radiosensitizing properties. Based on a small study evaluating PE with concurrent radiation that demonstrated a doubling of survival compared with historical data and the encouraging results with this combination in a Southwest Oncology Group (SWOG) trial in limited-stage small cell lung cancer, PE was chosen for subsequent phase III studies. Trials evaluating second-generation agents (taxanes, vinorelbine, gemcitabine, and irinotecan) in combination with cisplatin or carboplatin concurrently with radiation were also conducted. Weekly paclitaxel and carboplatin emerged as a well-tolerated and efficacious regimen. The most recent phase III randomized trial evaluating the modern regimen pemetrexed and cisplatin followed by pemetrexed consolidation versus standard chemoradiotherapy with PE in patients with nonsquamous histology was stopped early for futility ( Table 3 ) after randomization and treatment of 555 patients. OS for the pemetrexed and cisplatin arm was found not superior to the PE arm. As a result of these studies, concurrent weekly paclitaxel and carboplatin or cyclic PE remain the most commonly administered regimens.





