Surgery remains the only potentially curative therapy in the management of localized adult soft tissue sarcomas and gastrointestinal stromal tumors. There are over 50 different unique histologic types of soft tissue sarcomas, with different patterns of recurrence and prognosis. Surgical principles and sensitivity to locoregional and systemic treatments vary considerably based on the histologic type and anatomic location, as discussed in detail in this review.
Key points
- •
Sarcomas may arise in a variety of body sites and within a variety of tissues. The management of soft tissue sarcomas (STSs) and gastrointestinal stromal tumors (GISTs) requires a thorough understanding of the biology of the different histologies and molecular subtypes as well as the constraints of specific anatomic site.
- •
Limb-sparing and function-sparing approaches should be used when feasible for STSs located in the extremities and girdles, but extent of surgery should not be compromised for ease of closure. Margins of resection and use of adjuvant/neadjuvant radiation therapy and chemotherapy are contingent on accurate histologic diagnosis. Adjuvant therapy after a marginal resection, however, is not an appropriate substitute for a margin negative operation.
- •
Extended resections, including adjacent viscera, which may be adherent but not invaded, should be the goal in retroperitoneal sarcoma (RPS), to minimize microscopic intralesional margins and maximize local tumor control and possibly improve survival. The use of neoadjuvant radiation therapy is under investigation. Adjuvant (postoperative) radiation therapy is of limited value. Chemotherapy is not routinely used, save for specific sensitive subtypes.
- •
Complete tumor resection avoiding tumor rupture should be the goal in GIST. Preoperative imatinib should be considered whenever the expected morbidity is not minimal or surgery is not expected to be microscopically complete.
- •
Treatment planning should include multidisciplinary consultation to determine optimal therapy, taking into consideration tumor histology, site, and extent of the disease; its natural history and sensitivity to available treatments; surgical challenges; and the wishes of patients.
Introduction
Surgery remains the standard and only potentially curative therapy in the management of localized STSs of the adult and GISTs. Although belonging to the same family of tumors, surgical principles and sensitivity to locoregional and systemic therapies are completely different between the 2 entities; therefore, they are discussed separately. The treatment of pediatric sarcomas (including primitive peripheral neuroectodermic tumors, alveolar/embryonal rhabdomyosarcomas, and desmoplastic small round cell tumors) is different; systemic chemotherapy is the primary treatment and surgery may sometimes be omitted. The discussion of pediatric sarcomas is beyond the scope of this review.
Introduction
Surgery remains the standard and only potentially curative therapy in the management of localized STSs of the adult and GISTs. Although belonging to the same family of tumors, surgical principles and sensitivity to locoregional and systemic therapies are completely different between the 2 entities; therefore, they are discussed separately. The treatment of pediatric sarcomas (including primitive peripheral neuroectodermic tumors, alveolar/embryonal rhabdomyosarcomas, and desmoplastic small round cell tumors) is different; systemic chemotherapy is the primary treatment and surgery may sometimes be omitted. The discussion of pediatric sarcomas is beyond the scope of this review.
Soft tissue sarcomas
The basic principle for surgery is that the tumor must be resected en bloc with a cuff of healthy tissue, to avoid contamination of the residual tissue from the tumor surface and remove tumor microsatellites, which may be present in the healthy tissues surrounding the pseudocapsule. The extent of resection and adequacy of margins depends on a variety of factors, including histology and presence of an intact biologic barrier, such as muscular fascia, vascular adventitia, periosteum, or epineurium. Every attempt should be made to avoid positive microscopic margins (tumor at the border of the specimen), because this is independently associated with a worse survival.
Patients with STS should undergo evaluation at a sarcoma center, because the administration of neoadjuvant or adjuvant therapy may be indicated. Generally, this involves multidisciplinary evaluation by a surgical oncologist and, depending on histology, a medical oncologist and a radiation oncologist. Re-evaluation of pathology slides by a pathologist specializing in sarcomas is critical, to confirm the exact histology and determine the most appropriate treatment. Approximately 24% of all sarcomas are initially designated with the incorrect histology, usually by pathologists who do not have extensive experience with evaluating sarcomas, and approximately 16% are assigned the wrong histology in a clinically significant manner, having an impact on treatment plan. STSs are a family of more than 50 different diseases that can arise anywhere in the body. Principles of local treatment may vary according to histologic subtype and site. Surgical margins that may be adequate for one histologic subtype may be inadequate for another, and the sensitivity to and goals of chemotherapy and radiation therapy vary considerably between different histologies.
Histology-Specific Treatment
Atypical lipomatous tumor/well-differentiated liposarcoma
Atypical lipomatous tumor (ALT) and well-differentiated liposarcoma (WDLPS) are histologically the same entity. By convention, the term ALT is used when referring to low-grade lipomatous neoplasms confined to the trunk or extremity, where margin-negative resections are possible, and WDLPS is used when referring to deeper cavity tumors in the abdomen, retroperitoneum, and chest, where margin-negative resections are generally not possible. When arising in the extremity, this tumor has a low rate of recurrence, may not recur for some time, and has no risk of distant metastatic spread and death, unless dedifferentiation occurs over its natural history. Dedifferentiation, if it occurs, entails a risk of metastatic spread as high as 20%. In contrast, low-grade, locally recurrent ALT may grow slowly for years. Therefore, such tumors arising in the extremity can be resected with a limited negative or even a positive margin, especially when preserving limb function is an issue. Radiographically, ALT may be difficult to distinguish from an intramuscular lipoma, a benign entity that can also arise in deep muscle tissue. WDLPS is a more threatening neoplasm when located in the retroperitoneum, even in absence of areas of dedifferentiation. As discussed later, local control is a challenging issue at this site, and patients often die of locoregional failure, without developing distant metastases.
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma protuberan (DFSP) is a superficial tumor that infiltrates soft tissue for centimeters beyond the obvious margins of the lesion and can recur locally after an inadequate resection. The more common variety of DFSP does not display metastatic behavior. Therefore, the goal of surgery should be negative margins, often necessitating reconstruction by plastic surgery. Once negative margins are obtained, the risk of failure is virtually none. When cosmesis is an issue, limited positive margins may be accepted, and a wider resection postponed until DFSP locally recurs. Moreover, a limited positive margin does not automatically translate into local recurrence and does not increase the risk of metastatic spread. Radiation therapy (described later) is not routinely considered, even in the presence of positive margins. Approximately 5% to 10% of patients with DFSP have a more aggressive fibrosarcomatous variant, which may recur locally and metastasize. Those individuals should be treated as patients affected by a conventional sarcoma with more aggressive local therapy (including radiation) and followed with systematic imaging.
Myxofibrosarcoma
Myxofibrosarcoma, when located superficially, infiltrates through soft tissue (subcutaneous fat and investing fascia) centimeters beyond the ostensible margins of the visible or palpable mass. When located intramuscularly, the extension of the infiltration is usually limited by anatomic barriers, although it has a higher propensity to invade into those anatomic boundaries compared with other histologic subtypes. Myxofibrosarcoma most commonly arises in the extremities of elderly individuals. It demonstrates a 30% rate of local recurrence and 16% rate of distant recurrence. Multiple local recurrences have been associated with eventual amputation. Therefore, it is critical to pursue aggressive local therapy. Wide surgical margins (2–4 cm radial margins beyond the clinical boundaries of the palpable mass, especially in more superficial tumors) should be the goal of surgery, which often requires complex wound closure or flap reconstruction by a plastic and reconstructive surgeon as well as resection and reconstruction of vessels and/or nerves. Radiation therapy, either preoperatively or postoperatively (described later), may be considered, although the direct impact on this specific histology remain unknown.
Angiosarcoma
Scalp angiosarcoma is a particularly insidious malignancy. Scalp angiosarcomas are commonly multifocal, by both clinical examination and CT or MRI imaging. Although radical surgery is possible (requiring complex flap reconstructions), it is not uncommon for patients to develop local recurrences immediately outside the margins of resection even if the margins of the initial resection were widely negative and with or without radiation therapy. Angiosarcoma is sensitive to systemic chemotherapy and to radiation therapy. Because surgery is rarely curative, it should not be considered first-line therapy for scalp angiosarcoma. Surgery may be reserved for patients who are experiencing problems with local control (bleeding from a fungating tumor) or who only appear to have a solitary site of disease by both clinical examination and imaging while undergoing systemic therapy. Similar considerations apply to angiosarcoma arising at other superficial sites. When it occurs in the deep tissues, multifocality is less of an issue and the outcome is usually dominated by distant spread. The approach includes chemotherapy, radiation therapy, and surgery (discussed previously), although reconstruction by plastic surgery rarely is required. Vascular angiosarcoma has a particularly dismal prognosis and usually is also treated by a multimodality approach. For tumors not suitable to complete surgical resection, definitive radiation therapy, possibly with heavy particles, can be considered. Management of primary and radiation-induced (secondary) angiosarcoma of the breast is later.
Radiation-induced soft tissue sarcomas
Radiation-induced STSs are rare and include a variety of histologic subtypes, the most common of which are unclassified pleomorphic sarcoma, angiosarcoma, malignant peripheral nerve sheath tumors, and leiomyosarcoma. In addition to the intrinsic characteristics of each histologic subtype, they are all characterized by a high propenstity to locally recur, given the difficulty of obtaining clear margins. This is due in part to the difficulty in distinguishing tumor infiltration of healthy tissues from radiation-induced soft tissue changes around the tumor site and in part to the discontiguous and multifocal involvement of tissue within the radiation field. The tumor should be excised with as much tissue around it as possible. This often, if not always, requires reconstruction and coverage by a plastic surgeon and potentially a more liberal policy of neurovascular resection and reconstruction. Systemic chemotherapy and re-irradiation are often considered, given the overall dismal prognosis, although the use of the latter must be weighed with caution.
Malignant peripheral nerve sheath tumors
Malignant peripheral nerve sheath tumors tumors often arise from a major peripheral nerve, which can be identified macroscopically. They can occur sporadically or in the context of neurofibromatosis type 1 (von Recklinghausen disease). The high-grade variant is marked by an early propensity for distant metastases. When originating from a peripheral nerve, malignant peripheral nerve sheath tumors tumors also may spread along the nerve fibers proximally or distally. Wider margins at this level should be obtained (if possible at least 4 cm of macroscopic healthy nerve), in order to limit locoregional failure, which eventually may reach the spinal cord. Intraoperative frozen section analysis may help ensure clear margins. Systemic chemotherapy is used on an individualized basis.
Site-Specific Treatment
Extremity and trunk wall soft tissue sarcoma
Diagnosis
Imaging alone is rarely diagnostic of the specific sarcoma histology, with the exception of WDLPS (ie, ALT). ALTs have a radiographic density similar to normal surrounding fat but tend to be well encapsulated with thick internal septations. Such tumors may be treated with limited resection (complete excision with a minimal margin of normal surrounding muscle, fat, or fascia to minimize risk of local recurrence) and do not require a biopsy.
Other neoplasms suspicious for sarcoma should be biopsied. Core needle biopsy is the preferred method. It can be done either under radiographic guidance or without imaging directly in the clinic depending on location. Although biopsy tract recurrences are rare, the site selected for the core needle biopsy should be planned such that it can be included in the incision used during the subsequent definitive resection or at least in the radiation field. If a core needle biopsy fails to yield a diagnosis, an open incisional or excisional biopsy should be obtained. Although incisional biopsies commonly are used for diagnosis, they are often performed improperly in inexperienced hands. Incisional biopsies for extremity lesions should be performed through longitudinally oriented incisions placed such that the incision can be included in the final resection when a definitive resection is planned. If a transverse incision is used, then when subsequent re-excision is required, challenges for reconstruction and risk of lymphatic disruption (depending on location) are magnified. Excisional biopsies should be confined to lesions less than 2 cm in size and superficial in location.
Surgical treatment
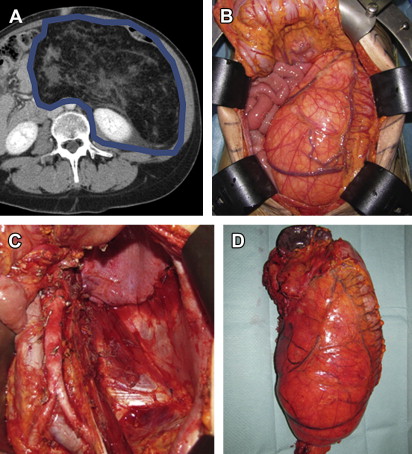
Currently, the goal of surgery is limb sparing and function sparing, while achieving appropriate biologic margins. Surgical resection should be carefully planned based on preoperative imaging. Resections should include not only the entire tumor (without rupture or violation of the surrounding pseudocapsule) but also an adequately wide margin (1–2 cm) of normal, non-neoplastic tissue ( Fig. 1 ). Resections performed with positive margins do result in higher risk of local recurrences and, to a lesser extent, of distant metastases and death ( Table 1 ). Tumors abutting bone may include the periosteum as a margin if the bone is not directly invaded. If necessary, vascular resection and reconstruction should be considered for involved vessels. If a critical nerve is encased, reconstruction with an interposition nerve graft should be considered. Such reconstructions may be compromised if radiation is administered postoperatively, and, therefore, preoperative radiation may be warranted. Furthermore, it is critical to take into account the specific histology (described previously). For instance, resection for myxofibrosarcoma requires wider margins than resection for ALT. Thus, it is important for surgeons to understand the different sarcoma histologies, confirm the accuracy of the diagnosis, and review the treatment plan in a multidisciplinary consultation.
| 5-y LR | 5-y DM | 5-y CSD | 10-y LR | 10-y DM | 10-y CSD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M+ (%) | M− (%) | M+ (%) | M− (%) | M+ (%) | M− (%) | M+ (%) | M− (%) | M+ (%) | M− (%) | M+ (%) | M− (%) | |
| Trovik et al, 2000 | 36 | 18 | 28 | 28 | NR | NR | NR | NR | NR | NR | NR | NR |
| Stojadinovic et al, 2002 | 35 | 18 | 32 | 24 | 30 | 20 | NR | NR | NR | NR | NR | NR |
| Zagars et al, 2003 | 36 | 12 | 25 | 28 | 31 | 25 | 44 | 14 | 33 | 33 | 39 | 34 |
| Gronchi et al, 2010 | 26 | 10 | 20 | 21 | 29 | 16 | 30 | 12 | 24 | 24 | 38 | 19 |
Adjuvant/neoadjuvant radiation therapy and chemotherapy
Limb-sparing surgery generally relies on adjuvant/neoadjuvant radiation therapy to minimize risk of local recurrence, as demonstrated in a landmark National Cancer Institute (NCI) trial. The goal of radiation is to treat the margin to minimize the risk of recurrence, not necessarily to reduce the size of the tumor per se. Radiation therapy reduces the risk of local recurrence from greater than 30% to less than 10% in most series but does not have an impact on distant failure or overall survival (OS).
Radiation therapy may be delivered as external beam radiation therapy (EBRT), brachytherapy, or intraoperative radiation therapy (IORT). EBRT may be delivered preoperatively or postoperatively. One randomized trial, by O’Sullivan and colleagues and sponsored by the Canadian NCI, compared preoperative EBRT to postoperative EBRT. There was no difference in local recurrence rates. Preoperative EBRT was associated with a doubling in the rate of wound complications (35% vs 17%) but importantly with a lower rate of late complications and tissue fibrosis and better functional outcomes. Postoperative EBRT generally covers a larger field (including drain sites) and is higher dose than preoperative EBRT. This is particularly important in young adults of childbearing age with proximal thigh STS; preoperative EBRT may spare the gonads whereas postoperative radiation may not. Moreover, the implementation of preoperative EBRT in a multimodality approach to high-risk extremity STS has also been shown to improve overall oncologic outcome in large retrospective series. Thus, it is the preferred approach at many experienced centers. A recent retrospective study identified diabetes, tumor size greater than 10 cm, tumor less than 3 mm from the skin surface, and need for reconstruction as independent predictors of major wound complications on multivariate analysis. Therefore, in patients potentially at higher risk of wound complications, judicious use of neoadjuvant EBRT, generous resection of at-risk superficial soft tissue, and proactive wound care measures may reduce complication rates.
Brachytherapy, delivered through afterloading catheters placed across the tumor bed at the end of surgery, or IORT, delivered during surgery via a cone applicator to the tumor bed after tumor removal, are options usually reserved to deliver additional radiation to a precisely defined close margin (including neurovascular structures) with minimal treatment to surrounding tissue, particularly when further EBRT is no longer feasible. Both brachytherapy and IORT may be used selectively with acceptable toxicity, but neither is generally used alone with either preoperative or postoperative EBRT. Brachytherapy offers the advantage of awaiting final margin analysis before determine which margins are indeed close, because catheters are not typically loaded with radioisotope for a minimum of 5 days postoperatively. They are not considered as routinely as EBRT, especially when preoperative therapy has been delivered.
Patients with small (<5 cm), superficial, well-circumscribed STS resected with an appropriately wide margin (>1 cm) of non-neoplastic tissue or biologic barrier (fascia) may not require RT, provided that the patient can be reliably followed.
Approximately 25% to 50% of patients with extremity STSs develop distant metastatic disease. Those with large (>5 cm), deep, high-grade STSs may be considered for preoperative or postoperative chemotherapy, usually with active agents, such as doxorubicin and ifosfamide.
The benefit of such an approach is limited, however, because it can at best provide an absolute improvement of OS of 5% at 10 years, and the data are inconsistent. Therefore, there is no consensus among sarcoma experts about the routine use of chemotherapy in the adjuvant setting for extremity sarcomas. Nevertheless, recent data have shown that when chemotherapy is selected for high-risk STS patients, a short full-dose regimen consisting of 3 cycles of anthracyclines plus ifosfamide may be considered. Toxicity is limited. It can also be delivered preoperatively and combined with RT, depending on the surgical needs, especially for tumors of borderline resectability or when preservation of function is a goal.
Hyperthermic isolated limb perfusion (ILP) has been investigated in several institutions because treatment of patients with locally advanced STS in whom limb-sparing, function-sparing surgery may not be possible. This procedure involves placing vascular access catheters into the main artery and vein of the affected extremity and perfusing with high-dose chemotherapy (usually melphalan) and tumor necrosis factor α under hyperthermic conditions. Although safe in experienced hands, early and late complication rates of more than 20% have been reported. More recently, isolated limb infusion (ILI) has been evaluated. Similar to ILP, ILI circulates high-dose chemotherapy in an isolated extremity but in contrast is generally performed through catheters placed percutaneously (rather than through an open approach) and under hypoxic conditions. Although high rate of complete responses (15%–30%) and further limb-sparing procedures (80%) are achieved by ILP and ILI, no randomized trials have ever compared either technique over aggressive limb-sparing resection with EBRT for STS. Arguably, patients under consideration for ILP usually have locally advanced or multifocal STS and are not necessarily candidates for surgery with EBRT at first evaluation. ILP and/or ILI should be considered as potential therapy in appropriately selected patients, and eligible patients should be referred to centers where this therapy is available.
RPS
Clinical and diagnostic evaluation
Imaging alone is rarely diagnostic of the specific sarcoma histology, with the exception of WDLPS. As described previously, WDLPSs have a radiographic density similar to normal surrounding fat but tend to be well encapsulated with thick internal septations. Giant lipomas are identified only anecdotally in the retroperitoneum; therefore, any mass consisting of very well-differentiated fatty tissue should be considered a WDLPS and treated as such. These tumors do not require a biopsy. Other lesions suspicious for RPS should be biopsied. The authors prefer to obtain core needle biopsies under radiographic guidance. Biopsy tract recurrences are rare and do not need to be re-excised during definitive surgery. Furthermore, if preoperative EBRT is planned, the biopsy site is usually included within the radiation field.
Surgical treatment
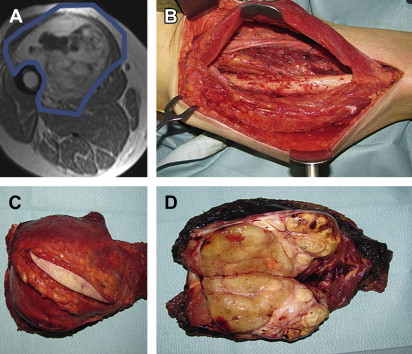
Proper resection of RPS requires appreciation of the anatomic boundaries of the tumor. CT imaging should be reviewed to identify landmarks defining the extent of the mass to determine which structures may be safely resected and which ones cannot. The anterior margin of an RPS is generally the ipsilateral colon and mesocolon, pancreas, liver, or stomach. The posterior margin is generally the psoas and iliacus muscles inferiorly, the ipsilateral kidney and diaphragm superiorly, and the ipsilateral ureter and gonadal vessels medially. These margins may vary, however, from tumor to tumor, and some or all of these structures could be anterior to the mass, in which case they would constitute a portion of the anterior margin. The medial margin usually includes the spine and paraspinous muscles, the inferior vena cava (for right-sided tumors), and the aorta (for left-sided tumors). The lateral margin is constituted by the lateral or flank musculoskeletal sidewall, although depending on the size and location of the tumor, the kidney and/or colon could also border the lateral portion of the mass. The superior margin is similarly dependent on the size and location of the mass and may include the diaphragm on either side, the right lobe of the liver, the duodenum, and the head/uncinate process of the pancreas for right-sided tumors and the pancreatic tail, spleen, and splenic vessels for left-sided tumors. The inferior margin may include the iliopsoas muscle; the femoral nerve; the common, internal, and external iliac vessels; and the pelvic sidewall. The size and specific location of the mass determine which of these many structures constitute which specific margin.
In general, the ipsilateral kidney, colon, mesocolon and at least a portion of the psoas can be safely and relatively easily resected. Resection of the, pancreatic tail and spleen can usually be performed with low short-term morbidity. Resection of other structures, including but not limited to the aorta, inferior vena cava, iliac vessels, femoral nerve, diaphragm, duodenum, pancreatic head or uncinate process, and liver, entail more significant resections, with ensuing greater morbidity.
The goal of surgery should be an aggressive multiorgan resection, removing involved or attached surrounding organs and retroperitoneal fat en bloc with the tumor in an effort to maximally clear the margins and avoid spilling tumor ( Fig. 2 ). This extended approach has shown to benefit patients affected by RPS, especially when the systemic risk was not high ( Table 2 ). A macroscopically incomplete resection is no more beneficial than nonoperative management.