Fig. 1
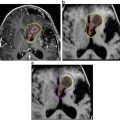
Staged therapy in a 10 year-old boy presenting with a large cystic craniopharyngioma (A). He underwent transsphenoidal decompression, with improvement in vision. The residual craniopharyngioma was evident on an MRI scan at three months post-operatively (B). This was subsequently resected via a right fronto-temporal craniotomy with very small tumor residuals which where adherent to the hypothalamus three months postoperatively (C)
Definitive Surgical Management
Current management of craniopharyngiomas is based upon an individualised risk-adapted strategy, where the extent of resection is tailored to the degree of hypothalamic involvement, as determined clinically and on pre-operative MR imaging. Although complete resection leads to optimal long-term disease control, the benign nature of these tumours and high survival rates mandate that low hypothalamic morbidity remains an important objective. The rarity and complexity of these tumours also requires that they be managed in institutions with enough experience and volume.
In the early series, the primary treatment objectives were preservation of vision and complete tumour removal [18–21]. Awareness of a significant number of recurrences despite documented complete resections, as well as insight into hypothalamic morbidity, led to a more conservative approach [22]. Accurate replacement of deficient pituitary hormones did not necessarily lead to a ‘normal’ life. In one of the early studies discussing the role of aggressive surgical management, 45 out of 50 children underwent gross total resection [18]. 34% recurred at a mean of 32.6 months. In addition, out of 28 children who were followed up in the long term, 16 had some impairment of memory, 24 were obese and 24 attended regular school, some requiring special help for cognitive and vision reasons. Over a 7-year follow-up, Sainte Rose et al. observed a 36% recurrence rate after previously confirmed ‘gross total resection’ [22]. In 70 children undergoing an attempt at complete resection by Yasargil et al., good outcomes were only reported in 60% [23]. In a series of 37 children treated at Boston Children’s Hospital between 1972 and 1981, none of the four patients who had undergone radical excision of their tumour and reached the end of high school, were able to work independently [24]. In another study involving long-term follow-up of 75 patients, 59 of whom underwent complete resection, 56% demonstrated evidence of hypothalamic dysfunction and in 16% this was severe [21]. Morbid obesity and chronically hyperosmolar children with an aberrant sense of thirst were seen almost exclusively after radical surgery.
Acknowledgement of long-term survival, increased understanding of the cost of hypothalamic morbidity , an awareness of a significant risk of tumour recurrence even after apparently complete resection, and an appreciation of the efficacy of radiotherapy at local control have led to the development of a tailored risk-adapted individualised strategy for this tumour. The relatively unknown long-term effects of radiotherapy, and the potential risks of managing recurrent tumours, dampen the enthusiasm for a universally minimal approach [22]. Most experienced units currently consider limited surgery only for those high risk cases where hypothalamic involvement is evident from pre-operative imaging and examination [9, 25, 26].
Operative Approaches
Selection of Operative Approach
The optimal choice of approach to adamantinomatous craniopharyngiomas remains controversial. However, there are a few rules of thumb, which are widely accepted by expert surgeons. Tumours of different size, location and extension require different strategies and individualised treatment concepts. As discussed above, there is unique agreement that a child who clearly exhibits signs of hypothalamic disturbance pre-operatively is not a candidate for extensive procedures such as attempts at total or subtotal excisions. These patients should only undergo procedures that are as minimally invasive as possible, and restricted to measures that decompress and clarify diagnosis.
The authors consider any patient with an intra- and suprasellar craniopharyngioma that is similar in location and shape to a pituitary adenoma an ideal candidate for transsphenoidal surgery; this is irrespective of whether a microscopic or endoscopic approach is used. The degree of sellar enlargement is the most decisive criterion on the suitability of a transsphenoidal approach. In contrast to the conventional, relatively conservative, indication for selection of the transsphenoidal route, those who favour extended endoscopic approaches see almost no limits to using this technique even for large lesions that are exclusively outside the sella. Enlargement of the sella by drilling the skull base bone and incising the dura until sufficient space is available allows dissection and resection of tumours located entirely outside the sella. The classification of Kassam et al. helps to find the ideally suitable patient, provided that sufficient experience with extended transsphenoidal approaches is available in the respective neurosurgical centre [27].
Most surgeons consider a fronto-temporal craniotomy and a pterional approach suitable for most suprasellar craniopharyngiomas, as long as their posterior extent does not expand beyond the clivus behind the dorsum sellae. For those who have a considerably retrosellar component, a fronto-basal midline approach, which requires dissection of the olfactory nerves, allows sufficient visualisation of the blood vessels of the posterior circulation, at least once the lamina terminalis is opened. Moreover, this approach is also suitable for tumours that have significant extension within the third ventricle. Transventricular approaches to the third ventricle are most appropriate for tumours which are entirely located within the third ventricle. Whether a lateral transventricular approach is used or a transcallosal paramedian route is selected depends mostly on the size of the foramen of Monro. However, every surgeon has his own preference and his own experience with the various approaches, and one usually selects a technique which one feels most comfortable with because of familiarity with the operative view.
Cyst punctures and tumour biopsies can be equally performed with frame-based or frameless navigation-guided procedures. Intraoperative MRI facilitates all surgical techniques , by demonstrating the extent of decompression or resection, and updating the image-guidance data set (Fig. 2).
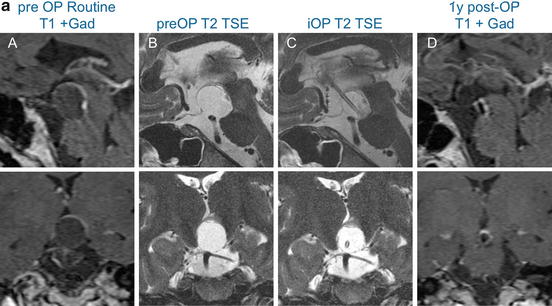
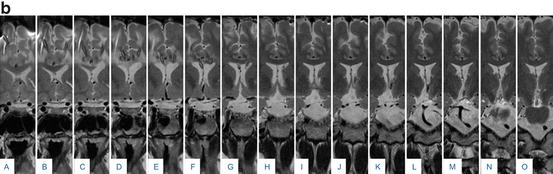


Fig. 2
(a) 29 year-old male patient presented with an increasing cystic remnant 10 years (September 2009) after bifrontal craniotomy for a adamantinomatous craniopharyngioma. Pre-operative contrasted T1 and T2 images are shown in (A) and (B). A cysto-ventricular drain was inserted and its tip position was controlled on intraoperative MR-imaging (C). The cyst was aspirated successfully, as demonstrated on a post-contrast T1 MRI scan one year later (D). (b) Standard post-operative serial coronal T2-weighted imaging (2 mm distance) demonstrates the catheter running through the left lateral ventricle (F–J) with the tip ending in the cystic residual craniopharngioma (N and O) above the pons. It serves as a continuous drainage of the content of cystic residual into the lateral ventricle. Additionally a right sided ventriculoperitoneal shunt is visible, (A–D); this was implanted prior to the bifrontal craniotomy due to obstructive hydrocephalus
Pterional Approach
The pterional approach is the most frequently used approach in some large series [23, 28]. This represents the shortest route to the suprasellar region and allows dissection of the tumour through the opticocarotid triangle and the prechiasmatic space. The lower and anterior third ventricle are accessed through the lamina terminalis. The primary limitations of this approach relate to poor visualisation of a retrosellar tumour component and the small perforating arteries within the opticocarotid triangle [28]. This approach is also particularly difficult in the context of a prefixed chiasm, or if the tumour extends posteriorly into the third ventricle beyond its anterior third. The approach remains excellent for small craniopharyngiomas; the subfrontal or anterior interhemispheric approach is preferable for larger lesions, particularly if there is a retrosellar component.
Early decompression of the tumour facilitates dissection at the plane between the capsule and the arachnoid. Division of the falciform ligament and opening the optic foramen protects the ipsilateral optic nerve during dissection. Residual tumour is most likely located below the ipsilateral nerve and this area may be inspected with a ‘dental’ mirror towards the end of the procedure.
Preservation of the pituitary stalk is challenging as it often blends into the posterior capsule of the tumour, often eccentrically to one side. As the tumour is debulked and the capsule is freed off Liliequist’s membrane posteriorly, the stalk may become visible as it courses towards the infundibulum behind the chiasm.
Subfrontal and Interhemispheric Midline Approach
In the bifrontal interhemispheric approach, a low craniotomy, involving the frontal sinus, allows minimal basal frontal retraction. Frontal lobe trauma has been shown to be a significant predictor of morbidity, both immediately after surgery and at long-term follow-up [21]. The olfactory nerves are microsurgically freed from the basal frontal lobes [28]. Opening the chiasmatic and interhemispheric cisterns, allowing time for CSF to egress and the frontal lobes to relax, allows exposure of the optic nerves, chiasm, proximal optic tracts, lamina terminalis, anterior communicating artery and both A2 vessels. Initial tumour dissection begins in the prechiasmatic region. Division of the lamina terminalis allows approach to the retrochiasmatic tumour. This is usually distended by tumour and can be divided safely. Division of the capsule and internal decompression of the tumour is followed by careful dissection of the capsule from the reactive gliotic layer or arachnoid membranes; perforating vessels must be protected. This exposes the retrosellar space, and allows dissection of the tumour from the premesencephalic space as well as the basilar artery and proximal posterior cerebral arteries. Dissection within the retrochiasmatic space carries a slightly increased risk of visual deterioration through manipulation of the optic chiasm [28].
Frontolateral Approach
The frontolateral approach represents a combination of the pterional and the unilateral subfrontal craniotomy. It offers a short distance to the suprasellar region and allows good visualisation of the anatomy within the basal cisterns. It allows early decompression through CSF release from the basal cisterns, reduces risk to the olfactory nerves and minimises injury to the basal frontal lobes which results from bifrontal retraction in the bilateral subfrontal approach. The head is fixed in extension and rotated 30 degrees to the opposite side [29]. After release of CSF from the basal cisterns and internal decompression of the tumour, the anatomical structures are recognised and their relationship to the tumour is evaluated.
Using this approach, tumour below the ipsilateral optic nerve often represents the most difficult dissection. Opening of the opticocarotid triangle facilitates complete removal in this region. When the chiasm is post-fixed and the optic nerves are long, dissection of the suboptic component of the tumour can be accomplished through the interoptic and opticocarotid spaces. When the chiasm is prefixed, dissection through the lamina terminalis is required.
The pituitary stalk may be identified as a thin, vascular structure attached to the capsule of the tumour and the diaphragma sellae, running behind the chiasm towards the infundibulum. It may be possible to dissect tumour from it.
Careful inspection of the tumour bed with the operating microscope is the principal method of verification of the extent of resection [29]. Endoscopic inspection and intraoperative MRI are useful adjuncts.
Transventricular and Transcallosal Approaches
These approaches are useful for craniopharyngiomas that extend into, or are almost entirely within, the upper third ventricle towards the foramina of Monro. In selected cases, this approach may avoid the need to violate the lamina terminalis or floor of the third ventricle, potentially preserving pituitary function. Neuronavigation is used to select and plan the optimal trajectory in relation to the position of the tumour.
Some authors prefer the transcortical route in the presence of large ventricles [30]. Resection can be achieved through a 1–2 cm corticotomy. The third ventricle is explored through the foramen of Monro. After opening the tumour capsule the core of the tumour is debulked in piecemeal fashion. A cottonoid placed in the posterior third ventricle prevents egress of blood towards and into the aqueduct. Caution needs to be exercised when peeling the capsule from the walls and floor of the third ventricle; the authors prefer to leave residual capsule if it appears tightly adherent on exploration [30]. If the tumour extends into the posterior third ventricle beyond the trajectory through the foramen of Monro, a 30 degree endoscope is useful to evaluate the extent of resection and facilitate additional resection. The primary concern with this approach remains a small risk of causing epilepsy, recently estimated at around 8% [31].
If the ventricles are small, the transcallosal approach is preferred. The tumour is dissected and resected through the foramen of Monro unilaterally; this avoids potential bilateral injury to the fornices. The foramen may be extended posteriorly by dividing the anterior septal vein and the tela choroidea, allowing access to the posterior half of the third ventricular cavity [32].
Transsphenoidal Approaches
Craniopharyngiomas typically extend to various levels from the sella through the suprasellar space posterior to the optic chiasm. This suggests that they are excellent candidates for the transsphenoidal approach, as their long axis lies along the axis of visualisation [33]. Formal comparison between transsphenoidal and transcranial approaches is difficult, as tumour morphology is highly variable and the surgeon’s personal experience and preference is particularly relevant. Published meta-analyses have reached different conclusions. One study concluded that transsphenoidal approaches have led to higher rates of complete resection and lower recurrence rates, as well as lower rates of post-operative diabetes insipidus [34]. Another study restricted to paediatric craniopharyngioma concluded that although transsphenoidal surgery is associated with higher resection rates and lower morbidity , baseline differences lead to preferred selection of one or other approach and generate bias which may itself account for the differences [35]. The traditional microsurgical approach has more recently been supplemented by the endoscopic approach as well as the development of extended transsphenoidal and cranial base approaches. It remains the belief of some authors that microscope-guided transsphenoidal surgery allows higher stereoscopic visualisation and maintains better surgical maneuverability and tactile feedback when dissecting delicate structures off a tough tumour capsule [36].
The microscopic transsphenoidal approach proceeds in distinct phases, from the nasal to the sphenoidal and sellar stage. After wide exposure of the sellar floor, the bone is resected to the planum sphenoidale anteriorly and to the cavernous sinuses laterally. The dura is opened and the pituitary gland may be mobilised inferiorly or laterally as necessary. For intrasellar craniopharyngiomas, tumour dissection and resection is begun inferiorly and laterally, allowing the more superior component to descend [37]. For suprasellar lesions, division of the diaphragm at the tuberculum allows visualisation of the tumour capsule behind the arachnoid. After tumour cysts are decompressed, the capsule can be carefully dissected from the optic nerve, chiasm and stalk. The interface with the hypothalamus needs very cautious dissection [37]. In the final phase, the CSF barriers are reconstructed, usually with a fat graft harvested from the abdominal wall and a pedicled nasoseptal flap based on the sphenopalatine artery [27].
The development of endoscopic and extended skull base approaches has led to a higher use of the transsphenoidal route for craniopharyngiomas beyond the sellar region. With the endoscopic approach, the opening into the skull base is tailored to the extent of the tumour, and may include not just the pituitary sella but also the tuberculum, the planum sphenoidale and the posterior ethmoidal air cells [38]. The dura is opened in the midline from the posterior aspect of the planum sphenoidale to a point anterior to the pituitary gland, through the coagulated intercavernous sinus. On opening the arachnoid, the tumour capsule is seen anterior and superior to the pituitary gland and below the chiasm. The capsule is opened and systematic internal tumour debulking is carried out. Cystic components are drained. After tumour decompression, the plane around the capsule is explored. The capsule may be tightly adherent to neurovascular structures, including the optic nerves and the hypothalamus. If it is not possible to develop an adequate plane, it would be best to leave parts of the capsule behind and avoid neurological injury. A combination of angled endoscopes, including 30, 45 and 70 degree scopes, are useful to inspect the tumour cavity and achieve maximal safe resection. As in the microscopic transsphenoidal approach, a CSF leak is averted by combinations of a fat and/or fascial graft and a nasoseptal flap, harvested in the nasal phase of the procedure.
Results
The variation in size and extent of craniopharyngiomas, as well as the multiple surgical options and lack of standardised clinical trials, render a study of surgical outcomes difficult. This is complicated by the fact that in most publications results pertaining to adamantinomatous and papillary craniopharyngiomas are not presented separately. Nevertheless, a number of meta-analyses have attempted to comprehensively present the important outcome measures related to local control as well as endocrine and visual outcome.
A large meta-analysis evaluated the efficacy of local tumour control with various treatment strategies [39]. From a total of 442 patients, reported on from 1990, including adult and paediatric age groups, who underwent tumour resection, gross total resection (GTR) was achieved in 256 patients and subtotal resection (STR) in 186. In 85 patients STR was followed by adjuvant radiotherapy. Two and five-year progression free survival (PFS) were similar for the GTR and STR and radiotherapy group (81 and 91% at 2 years, respectively, and similarly 67 and 66%). The five and 10-year overall survival (OS) rates were also similar (98 and 99% at 5 years, and 98 and 95% at 10 years). The authors conclude that the addition of radiotherapy to STR is a ‘reasonable’ approach to achieving local control while limiting the well-reported hypothalamic morbidity associated with radical resection.
In another systematic review restricted to paediatric craniopharyngioma a total of 109 studies detailed extent of resection and long-term follow-up, with recurrence data available for a cohort of 377 patients [40]. The study found no significant difference in 1 and 5 year PFS between those children who underwent GTR (89% and 77%, respectively) and those who underwent STR combined with radiotherapy (77% and 73%, respectively). STR alone was significantly worse than STR with radiotherapy; one-year PFS was 84% for STR and radiotherapy and 76% for STR alone; 5 year values were 73% and 43%, respectively [40]. Twenty-two patients in the study underwent biopsy only followed by intracystic chemotherapy; there was no difference in PFS between these patients and those who underwent STR and radiotherapy.
The importance of adjuvant radiotherapy has been emphasised in several publications. Iannalfi et al. conducted a large literature review including 43 studies and 1716 patients between 1990 and 2012 who underwent radiotherapy for craniopharyngioma [41]. The reported 10 year local control rates varied from 77% to 100% and the long-term toxicity of combined limited surgery and radiotherapy was less than that of radical surgery [41].
Although meta-analyses provide a good overall statistical overview, they do not reflect the heterogeneity in treatment philosophies evident in the published series. STR may mean different things to different surgeons, and is likely to represent a more extensive resection for surgeons who aim for a GTR [39]. The data is derived from series of self-reported outcomes and it is impossible to control for biases at meta-analysis level [39, 42, 43].
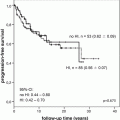
Prospective multicentre studies remain useful. Recent publications have reported on three-year follow-up of 117 pediatric patients recruited prospectively across centres in Germany, Austria and Switzerland [44, 45]. The reported three-year overall and event free survival were 97% and 46%, respectively; recurrence rates after complete resection were high, up to 36%, in 3 years [44]. Children who underwent irradiation reduced their risk of progression or recurrence by 88%. This study also observed a trend towards reduced surgical aggressiveness throughout its recruitment period. Children recruited within the second half of the study demonstrated far fewer hypothalamic complications (13% vs 35%) than those from the earlier half [45].
A recent study underlined the impact of minimising hypothalamic injury to overall outcomes in 65 children and compared them to a historical cohort of 59 children treated between 1985 and 2002 [46]. Surgical resection was complete in 60% of controls while 37% of children, recruited prospectively, underwent ‘hypothalamus-sparing’ surgery. Radiotherapy was given to 20 controls and 34 prospective patients. Post-operatively, patients who underwent ‘hypothalamus-sparing’ surgery had lower rates of obesity and endocrinopathies, particularly for corticotropic deficiency, gonadotropic hypogonadism and diabetes insipidus; there was no difference in growth hormone or thyroid stimulating hormone deficiency. The authors report no difference in the recurrence rate between the two groups, but acknowledge significantly shorter follow-up in the prospective group [46].
In a similar Canadian study, three cohorts of children undergoing treatment for craniopharyngioma between 1975 and 2011 were compared [47]. The frequency of GTR decreased over this period, whereas that of limited surgery, adjuvant radiotherapy and intracystic chemotherapy increased. Recurrence rates were not significantly different across the three time periods, changing from 34% and 30% in the first two decades to 52% in the last decade. There was, however, a significant reduction in the rate of diabetes insipidus (69% and 79% in the first two decades and 49% in the last decade) and ACTH deficiency (89% and 93% in the first two decades and 64% in the last decade). The prevalence of severe obesity decreased by 36%, although this was not statistically significant [47]. Similarly, in a combined review involving 122 adult and paediatric patients, the rate of local control with STR and radiotherapy was similar to those undergoing aggressive resection but the clinical outcome was superior [48].
Visual impairment is evident on presentation in more than half of children with craniopharyngioma [49]. Up to 50% of these improve post-operatively [35]. In a large meta-analysis that included adult patients, visual decline was reported in 3.7% of patients undergoing resective surgery and up to 8.6% in those who underwent radiotherapy alone [43]. This study also showed a statistical trend towards worse visual outcome in patients receiving radiotherapy after resection, compared to those undergoing resection, whether gross or subtotal, alone.
Children who have significant visual impairment on presentation and those with prechiasmatic tumour have a worse visual prognosis [35]. Mixed adult and paediatric series have demonstrated that visual prognosis is better in children [28]. In this series, post-operative visual deterioration did not occur in any of the 21 children who underwent transcranial surgery; however, across the whole series, this occurred in up to 15%. In this large series, visual outcome was best after transsphenoidal surgery [28]. This approach allows decompression of the tumour from below before any manipulation of the optic apparatus [50]. Elliott et al. found that in 86 children treated transcranially, the commonest new visual deficit, almost always transient, was contralateral homonymous hemianopia, related to dissection of the retrochiasmatic tumour component from a pterional approach [35].
Complications
Surgical complications are primarily related to the suprasellar location of craniopharyngiomas and include hypothalamic, vascular, cranial nerve, pituitary gland and stalk, as well as brainstem, injury. Although most complications are transient, craniopharyngioma and its treatment has a significant impact on children’s quality of life. Children with craniopharyngioma rated their health-related quality of life as considerably lower than healthy controls; the domains of social and emotional functioning were particularly affected [51]. Parents rated the impact of the disease higher than the children themselves.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree