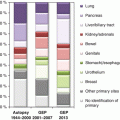
Resectable disease(“oligometastatic”)
Involvement of one lymph node area only (e.g., inguinal nodes), solitary lesions, resectable liver metastases
Cervical node metastases with squamous or undifferentiated histology
Strong resemblance to head and neck cancer
Axillary node metastases in women
To be regarded as node-positive breast cancer if not proven otherwise
Peritoneal carcinomatosis in women, especially papillary or serous adenocarcinoma
To be regarded as ovarian cancer or primary peritoneal carcinoma if not proven otherwise
Extragonadal germ cell tumors
Men, <50 years, undifferentiated carcinoma, retroperitoneal/mediastinal/pulmonary involvement, rapidly progressive, isochromosome i12p
Neuroendocrine tumors and carcinomas
NET I-II°, somatostatin receptor positive
NET III-IV°: resembling small cell lung cancer (SCLC)
Adenocarcinoma with colon-type histology
Immunohistochemistry positive for cytokeratin 20 and CDX2, negative for cytokeratin 7 (CK7−, CK20+, CDX2+)
Hormone-sensitive cancers
Expression of estrogen or progesterone receptor (ER+, PR+) mainly in women or androgen receptor or PSA in men
Non-carcinomatous-specific histology
For example, melanoma, sarcoma, lymphoma, etc.
8.4 Unfavorable Disseminated CUP
8.4.1 Single-Armed Phase II Trials
The first generation of treatment studies on CUP from 1964 to 1989 were mostly one armed and small and dealt with substances proven effective in other tumors including doxorubicin, 5-fluorouracil, mitomycin, methotrexate, vinca alkaloids, cisplatin, and cyclophosphamide [20–24]. Median survival ranged from a few weeks to 8 months. Therapeutic nihilism dominated the view of many treating physicians.
The second generation of treatment trials from about 1990 to 2003 were prospective, mono- or oligocentric phase II studies, most of which investigated only a single-treatment option. Obviously, one-armed trials in CUP are subject to considerable selection bias – the investigators simply will include patients whom they believe to benefit from this type of therapy while others are not included. Furthermore, favorable subsets (see Table 8.1) were not systematically excluded. These studies established a role for platinum compounds (mainly carboplatin), taxanes, and gemcitabine in the treatment of disseminated CUP with overall survival ranging from 7 to 13 months in selected patients [16, 25–28].
8.4.2 Randomized Clinical Trials (RCT)
Only RCT can minimize the major confounder in CUP treatment studies: selection bias. Unfortunately, randomized trials are scarce with less than 1000 patients included in all published trials to date (986 as of February 2015). Nevertheless, the first study was published as early as 1980 in the New England Journal of Medicine [29]. Several further first-generation studies followed (Table 8.2).
Table 8.2
First-generation randomized clinical trials
Author | N | Regimen | ORR (%) | PFS months | OS months |
|---|---|---|---|---|---|
Woods et al.(1980) [29] | 47 | C/M/FU MMC/Doxo | 4 36 | 1.7 4.5 | |
Eagan et al.(1987) [30] | 55 | MMC/Doxo MMC/Doxo/Cis | 14 27 | 5.5 4.6 | |
Milliken et al.(1987) [31] | 95 | MMC/Doxo Cis/Vbl/Bleo | 42 32 | 4.5 6.2 | |
Falkson andCohen (1998) [32] | 84 | MMC/Epi/Cis MMC | 50 17 | 9.4 5.4 | |
Dowell et al.(2001) [33] | 34 | Carbo/Eto Pac/FA/FU | 19 19 | 8.4 6.5 | |
Assersohn et al.(2003) [34] | 88 | FU cont FU cont/MMC | 12 20 | 3.6 4.1 | 4.7 6.7 |
Relevant results regarding modern chemotherapy treatment were added by the next generation of CUP trials (Table 8.3). These studies systematically excluded favorable subsets and were randomized, thus minimizing selection bias. One phase III trial – the biggest trial ever published – had to stop short of its aim and recruited 198 patients [36]. The remainder were randomized phase II studies with 80–100 patients included [14, 17, 37, 38]. Taken together, these trials built the basis of current first-line treatment of disseminated CUP with adenocarcinoma or poorly differentiated carcinoma. Platinum compounds, taxanes, gemcitabine, and irinotecan evolved as active compounds in CUP treatment. Tandem combinations outrivaled triple combinations because of comparable efficacy and less toxicity.
Table 8.3
Second-generation randomized clinical trials
Author | N | Regimen | ORR (%) | PFSmonths | OSmonths | Survivalrate |
|---|---|---|---|---|---|---|
Culine et al.(2003) [17] | 80 | Cis/Gem Cis/Iri | 55 38 | 8 6 | ||
Palmeri et al.(2006) [35] | 66 | Cis/Gem/Pac Cis/Gem/Vino | 49 42 | 9.6 13.6 | ||
Hübner et al. (2009) [14] | 92 | Carbo/Pac Gem/Vino | 24 20 | 6.1 3.2 | 11.0 7.0 | 38 % @1y 29 % @1y |
Hainsworthet al. (2010) [36] | 198 | Carbo/Pac/Eto Gem/Iri | 18 18 | 3.3 5.3 | 7.4 8.5 | 28 % @1y 35 % @1y |
Gross-Goupil et al. (2012) [37] | 52 | Cis/Gem Cis | 19 16 | 5 3 | 11 8 | 46 % @1y 35 % @1y |
Hainsworth et al. (2015) [38] | 89 | Carbo/Pac/Belino Carbo/Pac | 45 21 | 5.4 5.3 | 12.4 9.1 |
A recent meta-analysis of 1830 “unfavorable” CUP patients in 32 trials including 7 randomized trials showed a significant advantage of taxanes versus no taxanes and a nonsignificant trend of platinum vs. no platinum (Table 8.4, Ref. [18]). This meta-analysis on one hand suffers from the scarcity of robust data; on the other hand, its findings underline the efficacy of taxane and platinum compounds in the empirical treatment of unfavorable CUP.
N | OS | @1 year (%) | @2 year (%) | Significance | |
|---|---|---|---|---|---|
32 trials | 1830 | 9.0 | 36 | 19 | |
Carbo-/cisplatin No platinum | 1450 425 | 9.4 7.2 | 37 30 | 20 12 | N.S. |
Pacli-/docetaxel No taxane | 808 1067 | 9.6 8.3 | 41 31 | 21 16 | P = 0.03 |
Platinum + taxane No platinum + no taxane | 755 358 | 9.6 6.6 | 42 28 | 22 14 | P = 0.06 |
8.4.3 Squamous Cell Carcinoma
Squamous cell carcinoma is common in cervical lymph node metastasis with unknown primary but rare in unfavorable disseminated CUP, making up about 5–7 % of cases. Meaningful treatment trials do not exist – in contrast to squamous cell carcinoma affecting cervical nodes. Some groups simply include these patients into their adenocarcinoma and poorly differentiated carcinoma trials, but the numbers are too small to reach meaningful conclusions.
In effect, treatment recommendations extrapolate experiences of squamous cell carcinoma of known primary to situations with unknown primary. Cisplatin combined with fluorouracil is a common and quite effective combination, especially in regional disease combined with concurrent irradiation. Carboplatin/taxane combinations are a reasonable alternative, especially in patients with renal failure or reduced performance status. Based on results in non-small cell lung cancer, nab-paclitaxel may also be effective. Considering the efficacy of EGFR antibodies in the head and neck and non-small cell lung cancer as well as some adenocarcinomas, they may be an option in individual patients as well.
8.4.4 Colon Cancer-Like Treatment
Two trials report treatment that is effective in colon cancer as first-line therapy of disseminated CUP, applying capecitabine, oxaliplatin, and irinotecan [39, 40]. In these single-armed phase II trials, the study populations were dominated by patients with features hinting to a gastrointestinal primary though typical colon cancer-like histology (favorable subset) was excluded. The results were uniformly poor with progression-free survival of 2.5–3.5 months and overall survival well below 10 months. In second-line treatment, the combination of oxaliplatin and capecitabine performed as well as in the first-line setting (see Table 8.6). Taken together, colon cancer-like regimens are not indicated as first-line therapy in disseminated CUP. They may be an option in second-line treatment.
8.4.5 Second-Line Treatment
Only few small one-armed trials investigated the role of second-line chemotherapy in unfavorable CUP. These trials hint at a potential role of gemcitabine, irinotecan, oxaliplatin, and capecitabine as moderately active drugs after platinum-based first-line chemotherapy (Table 8.5).
Table 8.5
Second-line therapy
Author | N | Regimen | ORR (%) | PFS | OS | |
|---|---|---|---|---|---|---|
Culine et al. (2001) [41]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|