Epidemiology and historical trials in diabetic polyneuropathy
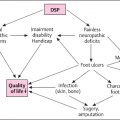
Diabetic neuropathy is the most common cause of distal symmetric polyneuropathy (DSP) worldwide . It generates the most morbidity and is linked to significant mortality rates of all diabetic complications . DSP is becoming more common with the global pandemic of diabetes and is expected to affect over 2.3 million in the United States alone . DSP is the leading contributor to nontraumatic amputations in the United States . DSP is also highly linked to diabetic autonomic neuropathy (DAN), which is a significant predictor of increased mortality in patients with both types of diabetes . DSP is a large source of loss productivity and disability in the United States. The cost of DSP is 11–14 billion in the United States alone .
Despite the high number of patients affected with DSP and its morbidity, treatment of DSP has been limited to management of symptoms and glycemic control, which benefits type 1 diabetes mellitus (T1DM) patients more than type 2 diabetes mellitus (T2DM) patients. Multiple trials of growth factors, aldose reductase inhibitors, and other agents have been tested without success in preventing further progression of DSP . It has been theorized that focusing on nerve conduction velocity (NCV) and sural amplitude as the outcome measure of most treatment trials may have been a limiting factor in determining efficacy . The evaluation showed the change of sural nerve amplitude in well-controlled T1 and T2DM patients was less than 1 µV per year . Evaluation of intraepidermal nerve fiber density utilizing minimally invasive punch biopsies may be more sensitive for the assessment of change in neuropathy over time. In addition, utilizing a model of capsaicin-induced denervation to evaluate nerve regeneration may be a more sensitive measure .
Multiple studies were performed on therapy based on pathogenetically linked treatments. The first wave of these studies was in aldose reductase inhibitors, based on the polyol pathway in which glucose is converted to sorbitol via aldose reductase, leading to cellular injury . Aldose reductase inhibitors also prevented change in sodium channel displacement, which may explain its effect on conduction velocity slowing .
The finding of improvement in NCV with aldose reductase inhibitors led to 13 large clinical trials of aldose reductase inhibitors sorbinul, ponalrestat, and tolrestat which were negative . The endpoint for all the trials was NCV which did not meet clinical significance in individual trials. However, there was a trend, but a pooled meta -analysis showed improvement in peroneal motor conduction velocity and median motor conduction velocity but not in sural sensory conduction velocity .
Thiocytic acid, an antioxidant also called α-lipoic acid (ALA), was found to be effective in several studies to help with pain but did not significantly improve NCV in the trials. The Neurological Assessment of Thiocytic Acid in Diabetic Neuropathy (NATHAN) study, which was a large, multicenter trial of ALA in the United States, found no significant change in NCV and NCS data in the placebo or treated groups, resulting in a negative result. However, there was a clinical improvement in the ALA treated group in the NIS-LL (Neuropathy Impairment Score-lower limb) score . There was also a significant improvement in pain control, for which ALA is still recommended.
In addition to aldose reductase inhibitors and ALA, growth factors were also studied in diabetic polyneuropathy (DPN) with limited success. Recombinant human nerve growth factor (rhNGF) was studied in a large 84 center randomized controlled study which revealed no significant benefit from rhNGF despite significant improvement in animal preclinical data . Increased pain, myalgias, and edema were seen in the treatment group. An angiotensin-converting enzyme inhibitor, trandolapril, was also studied in diabetic neuropathy but did not show significant improvement . Angiotensin-converting enzyme inhibitors have been shown to improve microangiopathy in animal models . A smaller study had been previously positive for quinapril in DAN and peripheral neuropathy .
Glucose control and diabetic polyneuropathy
The most successful treatment of DPN has been found to be glycemic control, and this was much more effective in T1DM patients. The Diabetes Control and Complications Trial (DCCT) study was the largest study to evaluate the effect of intensive glycemic control on the development of complications in T1DM. A total of 1441 patients were randomized to conventional versus intensive control and followed in the Epidemiology of Diabetes Interventions and Complications (EDIC) . The DCCT was stopped early when the participants in the intensive group had significantly fewer complications and rates of progression. The DCCT/EDIC studies also demonstrated the concept of “metabolic memory,” in which patients who had been randomized to conventional control had persistently worse outcomes over time despite later adherence to intensive glycemic control. Early treatment was shown to be most effective in reducing the rate of complications. The DCCT/EDIC study has also shown that in T1DM after glucose and age, hypertension, elevated triglycerides, and elevated LDL cholesterol were also significant risk factors for the development of DPN and DAN .
However, glucose control for T2DM is not as helpful in preventing DPN, DAN, and was actually in the ACCORD study linked to higher mortality in the intensive control causing the study to be stopped early . The UK Prospective Diabetes Study did not use a strict definition of DPN and had narrow windows between the two groups which might have decreased the ability to detect improvement in DPN in this study . However, the Veteran Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Study and the Veterans Affairs Diabetes Trial did not show improvements in DPN with intensive glucose control .
It has been theorized that the secondary metabolic factors in T2DM drive the lack of improvement from intensive glycemic control. Type 2 patients are typically older at the onset of diabetes and have years of prediabetes prior to diagnosis. Prediabetes in itself has been linked to polyneuropathy in itself . Obesity and chronic inflammation related to obesity is theorized to be yet another mechanism contributing to DPN in the T2DM population . DPN is typically detected much earlier in the course of T2DM and often at the time of diagnosis of diabetes mellitus. DPN in T2DM is also more often painful . Focus on factors beyond glucose in T2DM is crucial to improvement in DPN treatments. Future directions have included a focus on triglycerides, diet and exercise, and chronic inflammation .
Bariatric surgery has some promise in treatment of DPN. Several prospective studies have shown the benefit of bariatric surgery in reducing neuropathy scores after surgery . Care must be taken as bariatric surgery, especially roux-en-y bypass has a risk of peripheral neuropathy after surgery especially associated with vitamin B deficiency , and rapid weight loss can lead to foot drop rarely . Meta -analyses suggest an overall benefit of bariatric surgery in the treatment of DPN . This may be due to the reversal of multiple risk factors such as hypertension, elevated cholesterol and triglycerides in addition to glucose control . Bariatric surgery may also reduce inflammation which is associated with obesity and type 2 diabetes .
Treatment of pain in diabetic polyneuropathy
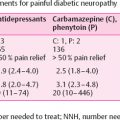
DPN is one of the most common causes of neuropathic pain and costs $11–14 billion US Dollars annually in lost wages, direct costs of health care, and disability. Painful DPN also contributes to increased incidence of mood disorders such as diabetes and anxiety . Currently, there are only four FDA approved treatments for painful DPN: pregabalin, duloxetine, tapentadol, and the new 8% capsaicin patch. All approach pain through different mechanisms. See Table 18.1 for the list of first-line medications for painful DPN .
| Medication | Typical dose range | Common side effects | American Academy of Neurology Level of Recommendation | Notes |
|---|---|---|---|---|
| Gabapentin | 300–1200 mg three times a day | Sedation, weight gain, peripheral edema | B | Adjust dose in patients with renal dysfunction |
| Pregabalin | 150–600 mg/d in two to three divided doses | Sedation, weight gain, peripheral edema | A | Adjust dose in patients with renal dysfunction; Schedule V controlled substance |
| Tricyclic antidepressants (e.g., amitriptyline, nortriptyline) | 10–25 mg at bedtime; titrate up to a maximum of 150 mg/d | Anticholinergic: dry mouth, constipation, orthostatic hypotension, urinary retention sedation, weight gain | B | Avoid in patients with a history of a prior suicide attempts; obtain ECG if titrating to high doses or if on concomitant QTc prolonging drugs. Also avoid in individuals with specific cardiac conductance abnormalities |
| Duloxetine | 30 mg/d to 60 mg two times a day | Nausea, dizziness, increased blood pressure, hyperhidrosis | B | Avoid in patients with hepatic dysfunction, and in patients with renal function with GFR below 30 mL/min. 60 mg two times a day has not been shown to be more effective than 60 mg/d |
| Venlafaxine | Immediate release: 37.5–225 mg/d in two to three divided doses once higher than 37.5 mg/d. Extended release: 37.5 mg/d; increase in 37.5 mg increments to total daily dose of 225 mg/d | Nausea, dizziness, increased blood pressure, hyperhidrosis | B | Extended release formulation may be better tolerated and less likely to be associated with a withdrawal syndrome on discontinuation |
Gabapentanoids
Pregabalin and gabapentin are both alpha2 delta calcium channel blockers. By blocking hyperexcitable dorsal root ganglion cells, gabapentanoids decrease neurotransmitter release of norepinephrine, glutamate, and aspartate . Gabapentin was originally designed as a GABA analog to treat epilepsy and was serendipitously found to treat neuropathic pain. Gabapentin is typically started between 300 and 900 mg daily in divided doses. The half-life is short, with peak concentration at 3 hours, requiring 3–4 times a day dosing. It also has limited bioavailability at higher doses, requiring more frequent dosing to maintain pain control. The most common side effects are sedation, weight gain, and peripheral edema. It is limited by renal dysfunction as it is renally cleared. It has the significant benefit of having minimal interaction with other drugs and can also treat restless leg syndrome (RLS), commonly seen in polyneuropathy patients . Pregabalin has a longer half-life, can be dosed twice to three times a day, and has a more linear bioavailability. It has the same side effects and limitations by renal function . It has a level A recommendation from the American Academy of Neurology’s (AAN’s) evidence-based guidelines for the treatment of painful DPN . Both drugs are Schedule V controlled substances limiting refills and requiring greater monitoring of abuse.
Serotonin norepinephrine reuptake inhibitors
Duloxetine was the first medication approved for DPN in 2004. It acts to inhibit the reuptake of serotonin and norepinephrine. By upregulating norepinephrine, it upregulates descending central pain control pathways using norepinephrine as the neurotransmitter . Duloxetine is also FDA approved for depression and is useful in patients with coexistent depression. It can be dosed daily, and the typical efficacious dose is 60 mg daily, although it can be increased to 120 mg daily. Typical side effects include nausea, increased sweating, hypertension, and decreased libido. It should be used with caution with other serotoninergic medications to avoid serotonin syndrome. It should also not be stopped abruptly to avoid withdrawal symptoms.
Venlafaxine has also been shown to be beneficial in neuropathic pain, although it is thought to be less effective than duloxetine. It has an extended-release formulation helpful in painful DPN with 32% reduction in pain at the 75 mg dose and 50% reduction at the 150–225 mg dose compared to placebo. It has similar side effects to duloxetine and the risk of withdrawal syndrome .
Tricyclic antidepressants
Tricyclic antidepressants were among the earliest medications used for neuropathic pain. Cochran reviews show amitriptyline has the lowest number needed to treat of all medications studied, 67% of patients experiencing benefit over placebo . However, the benefit is severely limited by side effects. In addition to targeting the same mechanisms as serotonin norepinephrine reuptake inhibitors (SNRIs), they also antagonize histamine and muscarinic anticholinergic receptors, increasing the side effect profile with sedation, dry mouth, constipation, urinary retention, and orthostatic hypotension. At high doses, they can also prolong the QT interval requiring ECG monitoring . This led to a level B recommendation from the AAN because of the high side effect and concern for treatment in elderly patients . Nortriptyline is also frequently used and has a similar side-effect profile. Both are dosed daily, typically at night, due to sedating side effects starting at 10–25 mg and titrating up to 150 mg/d maximum dose .
Antiepileptic drugs
Voltage-gated sodium channels have been implicated in neuropathic pain mechanisms and are thought to be hyperexcitable in painful DPN . Genetic mutations in sodium channel 1.7 gene SCN9A is known to cause erythromelalgia and insensitivity to pain . However, trials of epileptic drugs known to target sodium channels have led to minimally positive results. Valproic acid (VPA) has the most efficacy of all antiepileptic drugs (AEDs) tested in painful DPN . Not only does it affect sodium channels, but also potassium and calcium channels. It also has a number of significant side effects minimizing its use, including weight gain, thrombocytopenia, pancreatitis, tremor, sedation, hair loss, and significant teratogenicity. VPA is, therefore, typically a second-line treatment for DPN. Other AEDs used are carbamazepine and oxcarbamazepine, both sodium channel blockers and have been used for neuropathic pain in the past. A study of oxcarbamazepine initiated at 300 mg/d and titrated to a maximum of 1800 mg showed efficacy using a visual analog pain scale, but other studies were negative . Both oxcarbamazepine and carbamazepine can cause hyponatremia, and carbamazepine can cause bone marrow depression .
Another non-AED sodium channel blocker used is mexiletine, an antiarrhythmic agent, and used typically for myotonia. Mexiletine was not significantly better than placebo . Topical lidocaine has been studied for postherpetic neuralgia but has been used as an adjunctive agent .
Opioids
Opioids have also been studied in painful DPN. Tapentadol has been approved for painful DPN . It has a weak µ-opioid receptor antagonism and norepinephrine reuptake inhibitor activity. The extended-release formulation showed a greater than 30% efficacy over placebo, which led to its approval . It has relatively weak efficacy and, like other opioids, can lead to opioid-induced hyperalgesia . Oxycontin and morphine have been shown to be effective in placebo-controlled studies, especially in combination with other medications . However, their abuse potential and development of tolerance makes them a last-step option and typically, management is performed in pain clinics. Opioid-induced hyperalgesia is a phenomenon thought to be secondary to neuroplasticity and sensitization to µ-opioid receptors and nociceptor toll-like receptor 4, which may arise from opioid activation of mast cells, microglia, and other cells. Opioid-induced hyperalgesia causes increased pain and allodynia. It typically occurs with higher potency analgesics . The treatment is to recognize it, taper opioid treatment, and determine whether to restart after a holiday.
Patients with refractory neuropathic pain should be screened for depression, as overlapping depression can increase catastrophization and minimize benefits from medications . A multimodal pain clinic may be helpful for these patients. Although typical dogma is to raise an existing medication to higher doses before the trial of a second medication, in neuropathic pain, patients often benefit from the utilization of two different medications with different modes of action to improve pain control and minimize side effects. Some initial work on phenotyping pain patients to identify the most likely successful agent to individualize therapy has been performed using quantitative sensory testing, which may also lead to more successful pain treatment .
Supplements and alternative treatments for painful diabetic polyneuropathy
α-Lipoic acid
ALA is an antioxidant studied initially for the treatment of DPN but was shown symptomatically to improve pain . It is often used as an adjunctive pain reliever. It may be attractive to patients who are reluctant to use conventional therapies and have few side effects. The most common adverse effects are acid reflux and nausea. Doses of 600, 1200, and 1800 mg/d were all found to be superior to placebo in pain relief and neuropathy symptoms and change score . The NATHAN study suggested patients with fewer comorbidities of hypertension, cardiovascular disease, or obesity were more likely to improve in NIS-LL .
Benfotiamine
Benfotiamine, a synthetic derivative of thiamine, has been studied to treat diabetic neuropathy with conflicting evidence. Thiamine is a cofactor for several enzymes important in glucose metabolism in the Krebs cycle, such as transketolase, pyruvate dehydrogenase, and α-ketoglutarate dehydrogenase. Their actions are important for the prevention of toxic metabolites . In animal studies, benfotiamine reversed structural changes in the nerve and reversed the accumulation of advanced glycation endproducts . Several studies showed possible improvement in pain or in neuropathy symptom score but did not improve overall nerve function compared to placebo in a study of type 1 diabetic patients .
Cannabidiol and cannabis-derived treatments
Cannabinoids bind to cannabinoid receptors, CB 1 in the brain and TRPV 1 , peroxisome proliferator-activated receptor γ (PPARγ) GABA receptors, and calcium channels . Cannabinoid products studied include cannabidiol oil, tetrahydrocannabinol (THC) and cannabidiol nasal spray, nabilone (synthetic mimic of THC), inhaler herbal cannabis and plant-derived THC but showed a modest benefit of 28% improvement compared to 22% in the placebo group and were typically of small numbers . More data are needed before cannabinoids can be recommended for painful DPN.
Other supplements studied have included acetyl- l -carnitine, thought to enhance mitochondrial activity, curcumin, nutmeg, St. Johns wort, B vitamins (Metanx), and Lion’s mane ( Hericium erinaceus ), fish oil (containing omega-3 fatty acids) in animal models, but clinical trial data are lacking .
Exercise and nonpharmacologic treatments
Exercise is a promising therapeutic option for neuropathic pain and may effectively treat DPN itself . Animal exercise studies demonstrate increased nerve growth factor production in the dorsal root ganglion, including brain-derived neurotrophic factor and decreased pro-inflammatory cytokines such as tumor necrosis factor-α and IL-1β . Moderate aerobic exercise, in addition to strength training, has been studied, showing benefits in pain and intraepidermal nerve fiber density . Exercise may also be helpful in the treatment of DAN . Yoga has been studied in chronic pain but not specifically in painful DPN . Tai chi has been studied to help improve balance and decrease fall risk .
Acupuncture has been studied in several small studies but it has been challenging to develop a rigorous clinical methodology to prove efficacy . Limited insurance coverage and limited availability in some areas minimize its potential benefit. It is not clear whether acupuncture activates descending pain control pathways or decrease neuroinflammation.
Spinal cord stimulation has been studied in a few studies with patients having at least short-term pain benefits . Surgically laced electrodes in the epidural space produce electrical stimulation of nerve roots or dorsal columns theoretically block pain perception without ablation. Short-term cost is high, and risks include infection, damage to nerve roots or cord during surgical placement, and hardware failure.
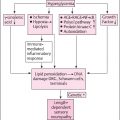
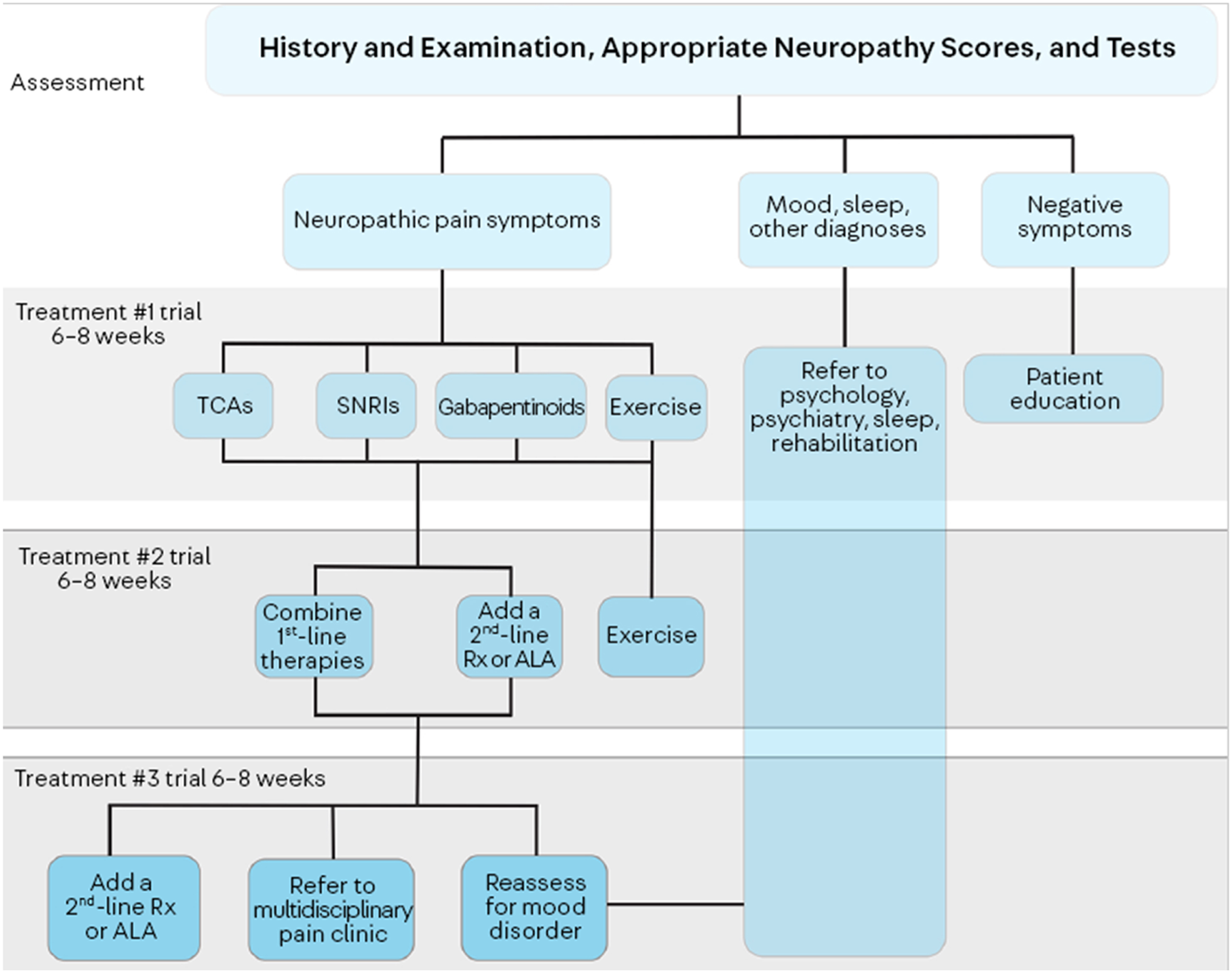
A typical algorithm for pain management includes using a gabapentinoid or SNRI as a first-line agent, and the choice depends on comorbidities such as RLS, depression. Many patients require more than one agent, and unlike standard pharmacotherapy where the first agent is pushed to maximum dose before the trial of a second, in pain control, usage of two agents may synergistically improve pain by acting on multiple pathways. All patients should be counseled on the potential benefit of exercise. If first and second-line agents are not helpful, referral to a multidisciplinary pain clinic may be necessary. Screening for depression is also key, as depression may lead to pain catastrophization and impaired sleep, increasing pain and minimizing response to pain management ( Fig. 18.1 ).
Emerging treatments and recent trials
Nav1.7 channels have been well documented to be involved in the development of neuropathic pain . Diabetic neuropathy models have shown an upregulation of Na1.7 channel subtype in painful diabetic neuropathy, possibly from TNF-alpha induced upregulation . A trial of a Na1.7 sodium channel blocker in painful DPN did not lead to superior pain relief over pregabalin, but further studies may be helpful .
Trial of stromal cell-like mesenchymal products was also studied in diabetic neuropathy. Human placental-derived adherent cells are a mesenchymal stromal cell-like population found in animal models to decrease neuroinflammation pain and promote angiogenesis . However, a phase 2a study of injection of placental-derived adherent cells did not significantly improve or prevent the decline of intraepidermal nerve fiber density. Angiotensin 2 receptor antagonists were also recently studied with negative results .
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, specifically the NADPH oxidase, type 5 isoform restricted to humans, has been recently studied as a potential target for diabetic complications of retinopathy and nephropathy and may also be a potential new target for treatments of diabetic neuropathy .
Conclusion
More work is needed in the treatment of DPN, which is currently limited to mainly pain control. The development of improved animal models and therapeutic endpoints would be helpful for future therapeutic trials . Further efforts to better identify clinical trial participants and refine the definition of diabetic neuropathy will hopefully lead to more positive treatment outcomes .
Further work on mechanisms of pain in DPN will hopefully lead to improved treatment of neuropathic pain in DPN. Evaluation of potential genetic variants in ion channels may also lead to improved pain treatments. The development of multimodal therapies involving multiple pain pathways may reduce the morbidity and loss of function in patients with refractory neuropathic pain.
Utilization of nonpharmacologic therapies such as exercise needs more study and may act on multiple DPN mechanism. Treatments such as bariatric surgery affect multiple pathways in the development of DPN and may be more effective in the treatment of DPN, especially in type 2 diabetes where glucose control is less effective in the prevention of DPN.
References
1. England J.D., Asbury A.K.: Peripheral neuropathy. Lancet 2004; 363: pp. 2151-2161.
2. Coppini D.V., Bowtell P.A., Weng C., Young P.J., Sonksen P.H.: Showing neuropathy is related to increased mortality in diabetic patients – a survival analysis using an accelerated failure time model. J Clin Epidemiol 2000; 53: pp. 519-523.
3. Boyko E.J., Ahroni J.H., Smith D.G., Davignon D.: Increased mortality associated with diabetic foot ulcer. Diabet Med 1996; 13: pp. 967-972.
4. Organization W.H.: 2020.World Health Organization Available from https://www.who.int/diabetes/global-report/
5. Gordois A., Scuffham P., Shearer A., Oglesby A., Tobian J.A.: The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003; 26: pp. 1790-1795.
6. Kiyani M., Yang Z., Charalambous L.T., Adil S.M., Lee H.J., Yang S., et. al.: Painful diabetic peripheral neuropathy: health care costs and complications from 2010 to 2015. Neurol Clin Pract 2020; 10: pp. 47-57.
7. Calcutt N.A., Fernyhough P.: A brief introduction to the history and controversies of clinical trials in diabetic neuropathy. Int Rev Neurobiol 2016; 127: pp. 3-8.
8. Bril V.: The perfect clinical trial. Int Rev Neurobiol 2016; 127: pp. 27-41.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree