Introduction
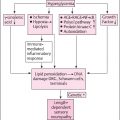
Diabetic peripheral neuropathy (DPN) remains a devastating microvascular complication of diabetes with current guidelines pointing to limited pharmacologic treatment options , mainly targeting symptomatic alleviation, optimization of glycemic control and improvement of cardiovascular risk factors . Although disease-modifying agents have been examined in clinical trials, clinically meaningful benefits were few or applied to restricted populations with special baseline characteristics . Hence, technology seems a modern and appealing strategy targeting alleviation of neuropathic symptoms, improved medical adherence, personalized offloading, and monitoring of physical activity . Furthermore, exercise has recently emerged as an additional potential therapeutic option , especially in the context of prior evidence pointing to association of the other diabetic microvascular complications, but not DPN, with exercise capacity . Intriguingly, exercise can be favorably combined with technology, but the efficacy of this combination remains to be proven . Finally, alternative treatments are now being increasingly appreciated, especially due to their more holistic, individual, and preventative nature .
Technologies
Technologies targeting improvement in quality of life and medical adherence
The contribution of new technologies to the treatment of DPN remains an appealing, albeit questionable issue. The recently published Diabetes Telephone Study has tested whether automated verbal communication between 1179 humans with symptomatic DPN at the point of beginning treatment might improve quality of life and contribute to alleviation of neuropathic symptoms . Interactive voice response technology (a total of three calls) over 6 months failed to prove beneficial . During a more intensified approach, individuals with painful DPN were randomized to receive either standard care or the latter plus twice-daily diabetes self-management text messages for 6 months . The latter aimed to provide education on diabetes, address lifestyle modifications, glucose monitoring and medical adherence, whereas 20% of them emphasized individual foot care. There were no significant differences in terms of pain and improvement of glycated hemoglobin (HbA 1c ), but text messaging was related to increased self-management activities and ameliorated diabetes-related health beliefs .
Technologies to identify high-risk for impendent diabetic foot ulceration
Prevention is better than cure in diabetes mellitus . Aiming to reduce recurrence of diabetic foot, artificial intelligence technology on mobile devices has recently been applied to automatically detect and localize diabetic foot ulceration (DFU) with a precision approaching 92% . In the same context, thermography is increasingly being appreciated as a further modality contributing to timely detection of incipient foot damage . Via implementation of thermography, high tissue temperature reflecting inflammatory response can be detected and monitored . Lavery et al. conducted one of the leading studies including 173 individuals with a history of DFU . Daily use of infrared skin thermometry on 6 foot sites for 15 months was compared with standard therapy alone. In the case of temperature differences >2.2°C (>4°F) between left and right corresponding sites, participants were instructed to immediately contact the study nurse and reduce ambulation . An almost fivefold lower risk for development of DFU was reported in the temperature monitoring group. These results were not replicated in a subsequent study .
To simplify thermometric evaluation, new devices have been developed, such as the smart-phone-based thermal camera and the wireless, sensor-embedded continuous temperature monitoring socks for use in the home environment . In a similar context , 129 diabetic persons with previously healed DFU were instructed to simply step on a wireless thermometric smart mat based on the telehealth concept, which could address plantar temperature variations. An asymmetry of >2.2°C (>4°F) between corresponding sites of the 2 feet soles correctly predicted pending DFU with 97% sensitivity but a modest specificity (<45%) . Of note, evidence supporting the cost-effectiveness of approaches implementing at home temperature monitoring is currently missing .
Technologies targeting personalized offloading
High plantar pressure has been traditionally recognized as a risk factor for the development of DFUs and, indeed, humans with history of DFU frequently exhibit high foot pressure . Hence, studies focusing on barefoot strain analysis have been conducted, the majority focusing on the forefoot and the assessment of ventrical plantar stress . Both barefoot peak pressure and peak plantar pressure above >200 kPa have been reported as independent predictors of future neuropathic DFU . Nonetheless, it seems plausible that site-specific plantar pressure may be more relevant in the prediction of DFUs. Indeed, in the study by Ledoux et al., increased baseline peak metatarsal plantar pressure was associated with a significant risk of subsequent metatarsal ulceration over a mean period of 2.4 years, but peak plantar pressure in distinct foot sites including the heel, midfoot, and the hallux was unable to predict DFU .
More recently, in-shoe analysis has been implemented to detect elevated plantar pressure. In a proof-of-concept study , dynamic in-shoe plantar pressure evaluation was proven an effective tool to guide footwear modifications leading to substantial reduction of the peak plantar pressure and an almost 30% pressure reduction among persons with DPN wearing custom-designed footwear.
Subsequent studies have highlighted the clinical significance of feedback approaches in the reduction of plantar pressure for the prevention of DFU . Feedback strategies implement mainly in-shoe technologies to warn of excessive plantar pressure (usually though visual or acoustic signs). They aim to make up for the loss of pain and to help humans with DPN toward suitable foot offloading during daily activities . The first outcomes of this method were controversial . Nevertheless, these preliminary studies included a very limited number of participants and applied a relatively short-term analysis of the intervention effects. In a subsequent study implementing wireless, pressure-sensitive insoles, an improved foot-off loading was reported at 6 weeks following training on a new walking strategy to reduce maximal peak pressure . This was based on pressure map analysis as obtained from insoles. Again, only half of steps were below peak pressure threshold . More recently, a prospective, randomized, multidisciplinary study assessed the efficacy of continuous, during daily activities, audiovisual alerts via a smartwatch linked to the insole system and offloading instructions among 58 DPN participants with a recent history of DFU . This intervention did not reduce the incidence of DFU overall. However, a subanalysis demonstrated almost 90% decreased incidence of DFU with the intervention among the 40 with adequate compliance .
Technologies to increase adherence to therapeutic footwear
Effective offloading of the diabetic foot is a prerequisite toward DFU healing . Nonetheless, evidence points to suboptimal adherence to therapeutic footwear. Indeed, the latter does not seem to exceed 30% of total daily activity . Even most compliant humans wear the offloading device no more than 60% of their daily activity . The home is probably further regarded as a comfort zone, with mean adherence approximately 60% . Therefore, technology has been implemented to enable objective monitoring of adherence. The temperature-based monitoring system attached to the inner surface of the device (footwear, upper limb or lower limb orthosis) was used to assess how long the device was used . Alternatively, it was combined with a step activity monitor to determine use during ambulatory activity . Adequate validity of this technology has been reported, but almost one-third of participants found the step activity monitor unpleasant . Similar technology has reported validity among healthy participants .
To further improve adherence, Najafi et al. implemented a 3-month smart insole system designed to prompt offloading in order to avert high plantar pressures . They attempted to prevent DFU among participants with prior neuropathic DFU . The device alerted the user through smartwatch auditory, vibratory, and visual feedback when “safe” pressure and time thresholds were exceeded. The procedure involved the user whenever there was an alert . High-pressure areas were identified via smartphone technology . Whenever participants applied offloading within 30 minutes of an alarm, this was defined as a successful response to the alert . A minimum number of alerts (one every 2 hours) was required to enhance adherence and to improve successful offloading .
Technologies to monitor physical activity
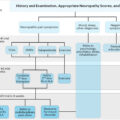
Early guidelines of the American Diabetes Association (ADA) discouraged walking and weight-bearing activities in DPN . The main concern was the susceptibility of the insensate foot to injuries and, thus the increased risk of DFUs . This approach changed with the study by LeMaster et al. in 2008 showing that weight-bearing activity does not increase neuropathic DFUs if proper footwear is worn. Accordingly, the latest ADA guidelines have been rephrased . Individuals with DPN are no more precluded from physical weight-bearing activity . However, several issues remain controversial, including optimal duration of exercise. Technology has now been employed to provide physical activity feedback ( Table 17.1 ). Following validation of the monitoring devices to detect weight-bearing activities , no association between physical activity and walking capacity was shown . Overall, studies ( Table 17.1 ) point to increased weight-bearing activity among humans who did not previously ulcerate . However, whether external ambulation exceeds internal is still controversial . Importantly, increased activity (≥7.5 hours/day) was reported to reduce the risk of recurrent DFU .
| Participants ( N ) | Feedback method (study duration) | Amount of activity reported | First author (year) |
|---|---|---|---|
| 100 | High-capacity computerized accelerometer/pedometer (minimum of 25 weeks or until ulceration) | Significantly lower average daily activity among individuals who ulcerated as compared to those who did not | Armstrong (2004) |
| 20 | Activity monitoring worn on the waistband, measuring number of steps taken over a period of time (1 week) | Restricted ambulation indoors, more external activity among humans with diabetes, neuropathy, deformity, or a history of lower-extremity ulceration or partial foot amputation | Armstrong (2001) |
| 30 | Two-dimensional accelerometer (15 days) | Humans with diabetes and a previous history of DFU were almost 50% less active as compared to nondiabetic humans but no difference was shown in comparison with diabetic humans without a previous history of plantar ulcer | Maluf (2003) |
| 400 | 24-hour physical activity questionnaire assessing daily weight-bearing activity (2 years) | Following adjustment for confounders, most active participants presented a significantly reduced risk of recurrent DFU as compared to the least active | Lemaster (2003) |
| 10 | Tri-axial accelerometer combined with GPS monitoring (3 days) | DFU participants’ weight-bearing activity was almost double indoors than away from home. At-home activity was similar between at-risk and active DFU participants. At-risk participants had 132% more external weight-bearing activity as compared to humans with active DFU | Crews (2017) |
Exercise
Quality of life, neuropathic symptoms, and deficits
Almost one out of 10 persons with diabetes mellitus experience debilitating neuropathic symptoms . Loss of sensation and increased vibration perception threshold represent important deficits of DPN, increasing the risk of DFUs . There is no consensus on improving neuropathic deficits . Thus lifestyle interventions might prove beneficial. A randomized controlled trial examined the effects of 8-week aerobic exercise of modest intensity on quality of life among 29 humans with DPN and 37 controls . Exercise significantly improved quality of life, pain scores, sensory symptoms, restriction in activity of daily life and disruptions in social relationships .
The same research group then examined the ability of this training intervention to improve vibration perception . Neuropathic symptoms were further assessed in the context of a randomized controlled trial of 31 DPN humans . These were assigned to either perform cycle training for 12 weeks (50%–70% of heart rate reserve, 30–45 minutes, 3 sessions/week) or to continue without training . A significant improvement of neuropathic symptoms (assessed with the Michigan Diabetic Neuropathy Score) was reported with exercise intervention . Moreover, exercise was associated with reduced HbA 1c .
Finally, in a clinical trial among persons >60 years of age with type 2 diabetes mellitus (T2DM), 12 weeks of plantiflexor and dorsiflexor muscle strengthening exercises with resistance bands, proprioceptive exercises on balance boards and a buoy combined with plantar sensory stimulation with bristle brushes and cloths significantly improved plantar cutaneous sensibility of both feet (as evaluated with Semmes-Weinstein monofilament) . Of note, limitations of this study include the lack of assessment of body weight parameters and glycemic control. Importantly, evidence form randomized controlled trials is only scarce , pointing to the need for future well-designed studies .
Physical capacity
Although previous evidence has implied that the level of physical capacity does not interfere with physical activity , improvement of the gait pattern is expected to optimize the functional outcomes of exercise without increasing DFUs . Thus a number of studies have addressed the association between exercise and improvement in physical status in DPN. In a randomized controlled trial , 12-week exercise for foot-ankle and gait training (40–60 minutes, twice a week) was associated with a significant improvement of the foot rollover process during gait. An improved eccentric control of forefoot contact, increased participation of hallux and toes, and increase in overall foot and ankle function were observed in comparison with the control group. Nevertheless, these effects were not sustainable during the following 12 months (24 months from baseline), highlighting the need for continuous training.
Similarly, simple home-based exercises were evaluated in 51 DPN humans, as compared with 53 controls . The intervention group was instructed to perform 10-minute exercises including eight exercises for both hands and four exercises for both feet, 3 times daily for 8 weeks . The exercise group significantly improved motor function (assessed with a special 5-point Likert scale) and specific activities of daily living, such as climbing stairs and performing household chores . In a more intensified approach, a 12-week group-specific progressive balance, flexibility, strengthening, and aerobic weight-bearing exercise (60 minutes, 3 times/week) was compared with the same nonweight-bearing training in a group of 29 participants with a mean age of 64.5 years . In this group of older DPN humans, weight-bearing activity significantly improved the 6-minute walk distance (between group difference reached 29 m) and daily step counts (between group difference was almost 1200 steps) . The clinical relevance of extra 1200 steps daily remains to be identified, but it appears important granted that persons aged between 55 and 95 years have a mean daily physical activity of almost 4000 steps/day .
Balance
Impaired balance represents an important risk factor for falls, especially among older DPN populations . Therefore a number of studies have examined the effects of exercise interventions on balance parameters and risk for falls in DPN and some systematic analyses have been conducted ( Table 17.2 ). The effects of a lower-extremity exercise and walking intervention (8 individual sessions with a physical therapist, plus exercises at home, 60 minutes, 3 times/week) were examined among 79 DPN participants with a mean age of approximately 65 years in randomized controlled trial . At the end of 12 months, intervention resulted in a small increase in the amount of time that participants could perform a one leg stand with their eyes closed . Conversely, there were no differences in other balance parameters or fall rates . However, this study was limited by a significant decline of lower-extremity muscle strength, pointing to an insufficient intensity of the intervention to provide benefit .
| Type(s) of exercise | Number of pooled trials (number of participants) | Eligibility criteria | Outcomes of interest | Results | First Author (year) |
|---|---|---|---|---|---|
| Systematic review and meta-analysis | |||||
| Aerobic exercise, resistance exercise, combination of both | 20 RCTs (1357 participants) | RCTs reporting effects of exercise on risk factors for DFU | DPN | The effect of exercise on DPN was not assessed—only one RCT | Liao (2019) |
| Mostly balance and lower limb strength training | 10 RCTs (889 participants) | Exercise interventions assessing falls-related outcomes among older diabetic humans | Falls-related outcomes (static and dynamic balance, lower limb strength, gait, falls rates, and falls risks) | Exercise interventions were more effective than the control condition for static balance, lower-limb strength and gait; no RCTs assessed falls-risk; one RCT reported 12-month falls-rate, with no differential treatment effect | Chapman (2017) |
| Systematic review | |||||
| Moderate- or higher-intensity exercise, resistance exercise, balance training, or a combination of exercise modes | 12 studies: 4 RCTs, 2 quasiexperimental studies, 1 one pre-post parallel group study, 5 pre-post single-group studies (5743 participants) | Effect of exercise on the progression and development of DPN | Nerve function (self-reported neuropathy scores, clinical nerve function tests, NCS, IENFD) | Eleven studies reported that exercise training had a positive influence on nerve function or neuropathy-related symptoms; one study reported mild adverse events (pain in the legs, knees, back of the hands with exercise, and pain/itchiness at the skin biopsy site) | Gu (2019) |
| Lower limb strengthening, balance practice, aerobic exercise, walking programs, and Tai Chi | 10 studies: 7 RCTs, 1 quasiexperimental study, 1 noncontrolled trial, 1 case-report (352 participants) | Effect of exercise training on falls risk among T2DM DPN humans | Falls risks | People with T2DM and DPN can improve their balance and walking after a targeted multicomponent program without risk of serious adverse events | Gu (2017) |
In another work, 8-week sensorimotor training, including wall slides, core exercises, balance exercises on unstable surface and gait training (65–80 minutes, 3 times/week), improved static and dynamic balance as well as proprioception measures among middle-aged and older humans . The risk of falls was not studied . Morrison et al. examined the ability of a 12-week moderate (heart-rate reserve 50%, 3 times/week, 45 minutes), or intense (heart-rate reserve 70%, 3 times/week, 30 minutes) supervised aerobic training to improve standing balance and risk of falls among 16 DPN humans and 21 humans with diabetes mellitus but no DPN (age range 42–70 years) . Following interventions, both groups significantly improved balance metrics, but the risk of falls was not reduced .
In a more focused approach, the effects of an 8-week balance exercise program (60 minutes, twice a week) were investigated among 38 DPN participants (mean age approximately 73 years) . Postural sway, trunk repositioning errors, dynamic and static balance significantly improved with the intervention . The risk of falls was not studied.
More recently, wearable technology has been combined with balance exercise (45 minutes, twice a week for 4 weeks) to improve postural stability in DPN humans (mean age 64 years) . Body-worn sensors were implemented to acquire kinematic data and provide real-time joint visual feedback during training . Significant improvement of postural balance was shown .
Nerve conduction parameters
Whether exercise is able to modify the natural history of DPN has been a matter of discussion. A 4-year supervised brisk walking on treadmill (heart-rate reserve 50%–85%, 4 times/week, 60 minutes) in DPN participants (mean age 49 years), led to a significant improvement of nerve conduction velocity of both peroneal and sural nerves and to a decreased risk of DPN development . Further evidence pointed to the favorable effects of aerobic exercise mainly on sural nerve conduction velocity of DPN humans but less so on peroneal nerve conduction . More recently, aerobic exercise, isokinetic strength and their combination 3 times/week for 12 weeks, were compared in a randomized controlled design among 45 DPN persons with a mean age >60 years . Regardless of the type of exercise, sensory (sural, median, ulnar) and motor (tibial and peroneal) nerve conduction did not improve . Finally, Hung et al. assessed the effects of exercise on nerve function in the diabetic hand exclusively , a rather forgotten diabetes complication . A 12-week Tai-chi Chuan exercise program significantly increased nerve conduction velocities but not amplitudes of the median, tibial, and ulnar nerves .
Intraepidermal nerve fiber density
Loss of small unmyelinated nerve fibers is traditionally deemed an early manifestation of DPN . Improvement through exercise has hitherto been unanimously supported by various training interventions . Indeed, the first study reported increased intraepidermal nerve fiber branching (+0.11±0.15 branch nodes/fiber) after 8 weeks of aerobic and strengthening exercises. These favorable changes were sustained at 12 months . However, it is now known that DPN may even occur in prediabetes . Smith et al. showed that 12-month lifestyle intervention (exercise plus diet) in humans with impaired glucose tolerance resulted in cutaneous reinnervation of both distal leg and proximal thigh, as well as reduced neuropathic pain .
Alternative treatments
Targeting balance
Despite promising molecules , there is currently an unmet need for improvement of treatment efficacy, for instance in balance. Foot plantar pressure massage (classic massage and deep friction massage at the right and left foot dorsum, medial, lateral regions of the foot, the toes, and the plantar region) for 10 minutes by a trained physiotherapist has been shown to improve balance immediately after the intervention . However, this study had no control group . Thai foot massage is another type of deep massage using thumb, finger, palm, or elbow pressure . It is applied along the meridian lines of the foot and leg and is combined with toe distraction . Chatchawan et al. examined the effects of 30-minute Thai foot massage, 3 days weekly in a randomized, parallel-controlled trial among T2DM participants with DPN . There was a significant improvement of balance after 2 weeks of Thai massage .
In a randomized crossover study in T2DM persons with DPN, a single session of self-applied Thai foot massage for 25 minutes was equally effective in terms of increasing the range of motion of foot and ankle to the same method applied by a massage therapist . Whole body vibration is another alternative intervention . A systematic review has shown its slight positive effect on both neuropathic pain and balance . However, it included very few studies and had a high risk of bias .
Targeting neuropathic pain
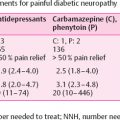
Neuropathic pain remains a major clinical concern, negatively affecting the quality of life . Nonpharmacological approaches, including electrotherapy, have been investigated for the treatment of painful DPN . Although the underlying mechanisms remain obscure, potential improvement of microcirculation and interference with pain gait control have been suggested. An initial study of frequency-modulated electromagnetic neural stimulation for a maximum of 3 weeks suggested a significant improvement of daytime and nocturnal visual analog pain score and quality of life, as compared with placebo. In this study, four electrodes administering monophase-compensated negative potential electrical pulses were applied to the lower extremities for 30 minutes and participants were required to discontinue all analgesics 3 weeks prior to study entry . Importantly, these beneficial effects persisted during a 4-month follow-up . Conversely, the same therapeutic intervention applied for 3 weeks in a double-blind, randomized, placebo-controlled clinical trial , significantly reduced daytime and nocturnal pain, but beneficial effects were not sustained at 3 months following the intervention. These two studies selected very diabetic populations: the former included participants with severe DPN , whereas the latter relatively mild symptomatic DPN .
It has long been known that hyperglycemia-related nonenzymatic collagen glycation may increase diameter and rigidity of ligaments and fascias . Thus surgical decompression of entrapped nerves in the lower extremities has been attempted to reduce neuropathic pain. Initial attempts that microsurgical decompression of the common peroneal nerve, deep peroneal nerve, and posterior tibial nerve resulted in >50% pain relief at 2 weeks postoperatively, with sustainable results even after 2 years . Favorable changes were also seen in physical capacity, social function, and mental health . Of note, a limitation of this study and previous reports was the failure to control for the nonoperated limb.
A further randomized controlled trial with a 12-month follow-up assessed the benefit of unilateral decompression of the tibial common peroneal, deep peroneal, and superficial peroneal nerves in painful DPN . Significant pain relief was achieved by approximately two out of five humans in the operated leg .
Noninvasive brain neurostimulation techniques have been used to inhibit pain perception in DPN . Daily application of this technique (deep H-coil repetitive transcranial magnetic stimulation applied via a helmet placed on the participant’s scalp at the point at which a minimum magnetic field was able to cause a motor response of the tibialis anterior muscle) for 5 consecutive days was well tolerated and significantly reduced neuropathic pain, as well as the threshold of RIII nociceptive flexion reflex , a measure of pain perception . Nevertheless, changes were not sustainable 3 weeks later, in line with a more recent study .
Furthermore, Reiki therapy was examined in a randomized, semidouble-blind, placebo-controlled 12-week trial . Reiki therapy is classed as a bioenergy field therapy . It is characterized as a spiritual practice without any religion references and involves a practitioner guiding energy to a receiver . Following 25-minute sessions over a 12-week period (twice weekly for the first week and weekly thereafter), no significant pain improvement was shown . However, all participants were permitted to continue analgesics and low pain scores were reported at baseline .
In addition, herbal products seem attractive, given that they have fewer untoward effects . A recent Cochrane database systematic review has included two studies reporting outcomes on both DPN and nondiabetic neuropathic pain . These assessed two herbal medicinal products, namely nutmeg nasal-spray (125 mL applied topically for 4 weeks) and St John’s wort capsules (900 mg total hypericin, ingested 3 times daily for 5 weeks) . The authors concluded that this evidence was insufficient to determine the efficacy of these herbal products for neuropathic pain alleviation . Finally, acupuncture therapy may improve pain of DPN, but relevant studies are characterized by varying methodologies and outcomes .
Discussion
Technologies have been used to identify persons at high risk of DFU. Evidence supports the benefits of home foot temperature monitoring , but this technology appears to provide the maximal benefit only in humans consistently wearing therapeutic footwear and its cost-effectiveness is unknown . Conversely, conflicting results have been achieved with technology in the improvement of quality of life in DPN . Furthermore, dynamic in-shoe plantar pressure evaluation has emerged as a promising approach to guide footwear modification, significantly reducing mechanical stress . Nevertheless, implementation of feedback visual or acoustic approaches targeting personalized offloading was not very consistent and was most beneficial in compliant persons . Of note, improvement of adherence to therapeutic footwear through technology is promising .
Exercise has also been reported to improve the quality of life and mitigate neuropathic symptoms . However, the role of improved glycemic control or use of additional modalities needs to be clarified, while evidence from randomized controlled trials is limited . Furthermore, the effect of exercise on physical status was overall beneficial , but continuous training was needed for sustainable benefits . Importantly, balance was improved with different exercise protocols among populations with different ages . Nevertheless, future research needs to examine whether falls may also be reduced. Surprisingly, exercise seems to also reverse small nerve fiber damage , even in prediabetes , while its effect on improvement of nerve conduction parameters (large nerve fiber function) is less certain .
Among alternative treatments, foot plantar pressure massage and Thai foot massage have improved balance, but studies were limited by short follow-up . Electrotherapy has yielded contradictory findings in terms of neuropathic pain reduction . It may be more effective in severe DPN . Surgical decompression of entrapped nerves has shown promising and more durable results . Noninvasive brain neurostimulation appears to offer only temporary benefit . Finally, Reiki therapy , herbal products and acupuncture have been investigated, but sufficient evidence of their efficacy has not yet accumulated.
Conclusion
DPN remains a frequently overlooked diabetes complication . In this context, technology provides modern possibilities, for instance foot temperature monitoring, dynamic in-shoe plantar pressure evaluation and implementation of feedback approaches targeting personalized offloading. Importantly, adequate compliance is a prerequisite. Furthermore, exercise emerges as an additional therapeutic option with beneficial effects even in prediabetes. Finally, alternative treatments provide additional option but we need long-term, well-designed studies.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree