Table 35.1 summarizes the pharmacokinetics of most commonly used insulin preparations.
Insulin regimens
Intensive therapy involves the administration of a basal level of insulin (isophane, glargine, detemir) and premeal boluses of a rapid-acting insulin preparation (lispro, aspart, glulisine). The administration of a basal long – acting insulin and boluses of rapid acting insulin with meals intends to mimic the normal insulin secretion profile of the pancreas. The basal insulin suppresses lipolysis and hepatic glucose production. The boluses of insulin minimize the postprandial rise in blood glucose.
For intensive insulin regimens, patients must be committed and be supported by an experienced diabetic team. Motivated patients can attend ‘DAFNE’ (Dose Adjustment for Normal Eating) courses to learn how to calculate their carbohydrate intake and adjust their insulin doses accordingly.
The use of premixed insulins (see ‘Insulin treatment in type 2 diabetes’) is not recommended for patients with type 1 diabetes. This is because intensive therapy in patients with type 1 diabetes requires frequent adjustments of the premeal bolus of the rapid-acting insulin.
Basal insulin is delivered by daily or twice daily injections of a long-acting insulin preparation, or by continuous subcutaneous infusion of a rapid-acting insulin preparation via a pump (‘continuous subcutaneous insulin infusion’). HbA1c values are similar in studies comparing isophane insulin with glargine. However, patients using glargine have fewer hypoglycaemic episodes. Insulin detemir must be given twice daily as it has a shorter duration of action than glargine.
Insulin injection devices, technique and sites
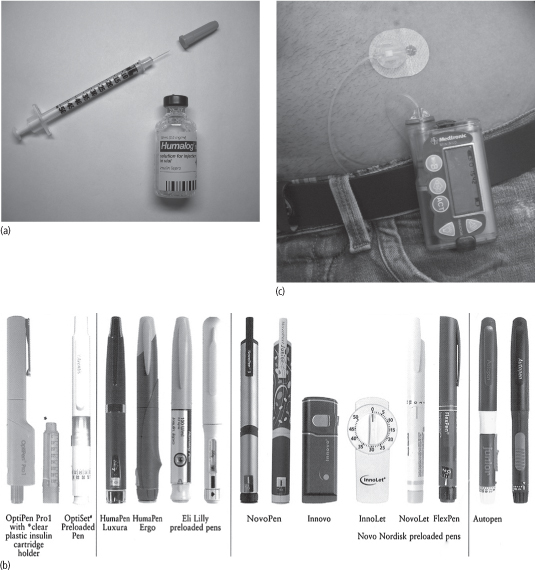
Insulin is injected subcutaneously using single-use syringes with needles, insulin pens with needles or an insulin pump (Fig. 35.1).
Insulin pumps may give slightly better glycaemic control. However, they are costly and cumbersome for some patients, and ketoacidosis may occur if the pump malfunctions. Patients choosing this regimen must have 24-hour access to expert advice.
Insulin pens may be prefilled (disposable) or reusable. Prefilled pens (e.g. FlexPen, InnoLet, OptiSet) are discarded when the insulin cartridge is spent. Reusable pens (e.g. OptiPen, NovoPen, Autopen, HumaPen) contain a replaceable insulin cartridge that is loaded into and removed from the pen by the patient.
Injection technique is the same with insulin syringes and with pens. Patients should learn the proper insulin injection technique from a diabetes specialist nurse. An area of the body in which about 2.5 cm of subcutaneous fat can be pinched between two fingers should be used. The needle (e.g. Micro-Fine 27G, Ultra-Fine 29G) is inserted perpendicular to the pinched skin up to the hilt. The length of the needle used depends on whether the patient is thin/child (5 mm), normal weight (8 mm) or overweight (12.7 mm). The needle is held in place for several seconds after insulin injection to avoid insulin leakage after withdrawal of the needle. Patients should be advised on the safe disposal of syringes and needles.
Potential sites for insulin injection are the upper arms, abdominal wall, upper legs and buttocks (Fig. 35.2). Insulin is absorbed fastest from the abdominal wall, slowest from the leg and buttock, and at an intermediate rate from the arm.
Figure 35.1 (a) Insulin vial and syringe. (b) Insulin pens. (c) Insulin pump.
The long-acting insulin preparations are best injected into the leg or buttock (from which absorption is slow). The rapid-acting insulin preparations are best injected into the abdominal wall (from which insulin is absorbed more rapidly).
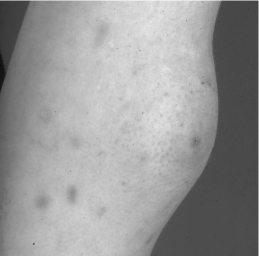
The injection sites must be rotated to avoid the risk of lipohypertrophy (Fig. 35.3).
Insulin dose
Insulin requirement depends on body weight, age and pubertal stage. Newly diagnosed children usually require an initial total daily insulin dose of 0.5–1.0 U/kg. Prepubertal children often require lower doses, and pubertal children may need higher doses. Patients in ketoacidosis and those receiving glucocorticoids also require higher doses.
Figure 35.2 Insulin may be injected subcutaneously into the shaded areas. Injection sites should be rotated.
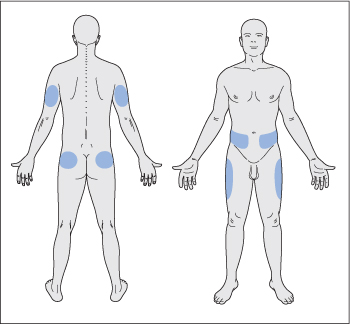
Figure 35.3 Lipohypertrophy caused by repeated insulin injections into the same site.
The basal insulin dose comprises about 50% of the total daily dose. The remaining 50% of insulin is given as rapid-acting insulin divided into three doses, administered before each meal. The boluses are adjusted according to the carbohydrate content of the meals and the current blood glucose level.
A period of decreasing insulin requirement may be seen in children and adolescents a few weeks after the diagnosis and initiation of insulin. This ‘honeymoon phase’ is due to a secretion of some endogenous insulin from the remaining functional beta cells and may last several months or occasionally years. Blood glucose must be closely monitored during this period. Hypoglycaemic episodes may occur if the insulin dose is not adjusted appropriately.
Side-effects
The main side-effects of intensive insulin therapy are hypoglycaemia and weight gain. Patients and their families should be educated to recognize and deal with hypoglycaemic episodes. The treatment of hypoglycaemia is discussed in Chapter 36.
Weight gain can limit patient compliance. Patients must pay attention to caloric intake and exercise to avoid the weight gain that commonly accompanies intensive insulin therapy.
Monitoring of control
Patients must monitor their blood glucose using a glucometer 4–7 times daily (before meals, mid-morning, mid-afternoon, before bedtime and occasionally at 3 a.m.). HbA1c should be checked regularly to confirm chronic glucose control (see above). Patients should be seen at least every 3 months, and must have access to as-needed telephone or e-mail consultations for insulin regimen adjustments.
Glycaemic control in type 2 diabetes
The United Kingdom Prospective Diabetes Study (UKPDS) showed that intensive therapy to achieve lower levels of glycaemia in patients with type 2 diabetes reduces the risk of microvascular complications (retinopathy, nephropathy, neuropathy). The 10-year post-trial monitoring data from the UKPDS show that early intensive glucose control reduces the risk of myocardial infarction and all-cause mortality as well as continuing to reduce the risk of microvascular complications.
The aim is to achieve normal or near-normal glycaemia with an HbA1c goal of below 7%. However, less stringent goals may be appropriate for older patients and patients with a history of severe hypoglycaemia, limited life expectancy or comorbid conditions.
In both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trials, intensive glycaemic control to achieve HbA1c levels below 6.5% in patients with longstanding diabetes did not reduce cardiovascular events over the time period studied (3.5–5 years). Data from the ACCORD study suggest that, in patients with a long history of diabetes and at high risk of cardiovascular disease, reducing HbA1c to near-normal may be unsafe (associated with a higher number of total and cardiovascular deaths, and a more common occurrence of severe hypoglycaemia). Therefore a target HbA1c of 7–7.9% may be safer for these patients.
HbA1c must be obtained at least twice yearly in patients who are meeting glycaemic goals, and every 3 months in patients who are not meeting glycaemic goals and in those whose treatment has changed.
Dietary modification and exercise are major components of non-pharmacological therapy in type 2 diabetes. Changes in lifestyle also slow the progression of impaired glucose tolerance to overt diabetes. However, a sudden initiation of vigorous exercise in sedentary patients can precipitate myocardial infarction. Therefore prior to starting an exercise programme, patients over age 35 years who have had diabetes for more than 10 years should be thoroughly examined, and an exercise stress test should be considered.
Patients should exercise regularly (at least three times per week) and preferably at the same time in relation to meals (and insulin injections). The American Diabetes Association recommends at least 150 minutes of moderate-intensity aerobic activity distributed over at least 3 days each week. Patients with proliferative retinopathy should avoid weight-lifting due to the increased risk of intraocular haemorrhage. Patients with neuropathy should avoid long-distance running or prolonged downhill skiing. These activities may precipitate stress fractures and pressure ulcers in the feet.
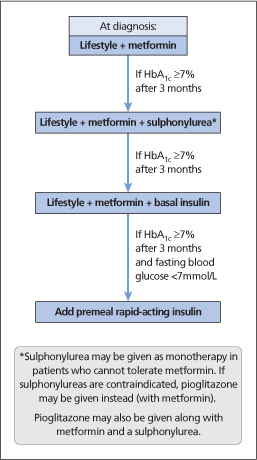
An overview of pharmacological therapy for glycaemic control in type 2 diabetes is summarized in Fig. 35.4.
Metformin
Metformin should be started at the time of diagnosis in patients with type 2 diabetes (if there are no specific contraindications), along with advice regarding lifestyle modification. Metformin may be started at 500 mg once daily with meals. It is titrated up over 1–2 months to the maximally effective dose (850 mg three times a day) as tolerated. A longer-acting formulation is available in some countries and can be given once per day.
Mechanism of action and efficacy
Metformin decreases hepatic glucose output, inhibits lipolysis (reduces serum free fatty acid levels and hence substrate availability for gluconeogenesis) and increases insulin-mediated glucose utilization in the peripheral tissues (muscle and liver). Metformin lowers serum lipid and blood glucose possibly by working through LKB1 (a protein-threonine kinase), which phosphorylates and activates the enzyme adenosine monophosphate-activated protein kinase.
Figure 35.4 Overview of pharmacological therapy for glycaemic control in type 2 diabetes.
Metformin is not associated with weight gain and typically reduces HbA 1c levels by about 1.5%. It is less likely to cause hypoglycaemia than sulphonylureas and insulin. It can cause a decrease in serum triglyceride (triacylglycerol) levels, a small decrease in serum low-density lipoprotein (LDL) cholesterol, and a very modest increase in serum high-density lipoprotein (HDL) cholesterol. In overweight and obese individuals with diabetes, metformin may reduce all-cause mortality and rate of myocardial infarction. Metformin has been suggested to reduce the risk of macrovascular complications, independently of its effects on glycaemic control.
Side-effects
Gastrointestinal side-effects (e.g. nausea, anorexia, abdominal discomfort, diarrhoea, a metallic taste in the mouth) are common with metformin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree