Stage I: Growth limited to the ovaries
IA: Tumour limited to one ovary; capsule intact, no tumour on ovarian surface; no malignant cells in ascites or peritoneal washings
IIB: Tumour limited to both ovaries; capsules intact, no tumour on ovarian surface; no malignant cells in ascites or peritoneal washings
IC: Tumour limited to one or both ovaries with capsule rupture or tumour on ovarian surface; malignant cells in ascites or peritoneal washings
Stage II: Tumour involves one or both ovaries with pelvic extensions
IIA: Extension or implants on uterus or tube(s), or both; no malignant cells in ascites or peritoneal washings
IIB: Extension to other pelvic tissues; no malignant cells in ascites or peritoneal washings
IIC: Pelvic extension with malignant cells in ascites or peritoneal washings
Stage III: Tumour involves one or both ovaries with peritoneal metastasis outside the pelvis or retroperitoneal or inguinal node metastasis
IIIA: Microscopic peritoneal metastasis beyond pelvis
IIIB: Macroscopic peritoneal metastasis beyond pelvis 2 cm or less in greatest dimension
IIIC: Peritoneal metastasis beyond pelvis more than 2 cm in greatest dimension or regional lymph node metastasis, or both
Stage IV: Distant metastasis (excludes peritoneal metastasis) to liver parenchyma or other visceral organs or a malignant pleural effusion
Diagnosis
Patients with advanced stage ovarian cancer may present with a variety of symptoms such as bloating, pelvic or abdominal pain, early satiety and urinary symptoms. There may be abnormal findings at pelvic or abdominal examination, or abnormalities found in imaging studies or surgery for other conditions. Ovarian cancer diagnosis may be made after a staging laparotomy for suspected ovarian cancer, image-guided needle biopsy, laparoscopic assessment and biopsy or biopsy during other operations. Cytology of ascites or pleural fluid is not recommended to make the diagnosis as they may not provide sufficient diagnostic accuracy to both confirm and classify ovarian cancer. Cases of suspected and confirmed advanced stage ovarian cancer should be discussed by multidisciplinary teams including a gynaecological oncology surgeon, medical or clinical oncologist, radiologist, pathologist, clinical nurse specialist and palliative care team with at least one member having assessed the patient. This multi-professional discussion will ensure that holistic management of patients can be planned and pathways of care organised which often involve a combination of surgery and chemotherapy alongside other hospital and community services.
Surgery in Ovarian Cancer
Surgery has been conducted for patients with ovarian cancer since the 1930s and in 1975 Griffiths et al. published a landmark paper demonstrating an inverse relationship between residual tumour diameter after surgery and survival [1, 2]. After the benefits of platinum-based chemotherapy (cisplatin and carboplatin) were established in the 1980s, there have been no randomised controlled studies to compare the outcomes of patients receiving and not receiving surgery in addition to chemotherapy and surgery continues to be performed. The timing of initial surgery may be before chemotherapy (primary surgery) or after chemotherapy (delayed primary surgery). Secondary surgery (a subsequent laparotomy) may be performed during chemotherapy (interval debulking surgery – IDS) or after completion of chemotherapy (second-look laparotomy). The roles of surgery include diagnosis, staging, palliation and cytoreduction.
In the platinum era the benefit of surgical debulking persisted. In Bristow’s meta-analysis of 53 studies including 6,885 patients with stage III or IV disease, for every 10 % in cytoreduction median survival improved by 5.5 % [3]. There is uncertainty whether this represents inherent tumour biology – or benefit gained from surgical aggression and radicality.
Cytoreduction is usually classified as complete when there is no visible residual disease at the end of surgery, optimal if there are only small tumour deposits at the end of surgery (usually <1 cm maximum diameter) and suboptimal if there is greater than 1 cm of disease after surgery (Table 10.2). As the definition of optimal debulking has changed over time and between studies, more recent studies have classified optimal as complete cytoreduction, suboptimal as <1 cm deposits [4]. The benefits of surgery may include (1) resection of areas of tumour that are poorly vascularised and in which chemotherapy may be less effective, (2) higher growth fraction in well-perfused small residual tumour masses that may be more chemosensitive, (3) treating a smaller volume of disease may need less chemotherapy and reduce the opportunity for resistant tumour clones to develop, (4) resection of drug-resistant tumour cells and (5) host immunocompetence improved with removal of tumour bulk [5]. Complete and optimal debulking rates are higher when patients are operated on by gynaecological oncologists in specialised hospitals, compared to general gynaecologists or general surgeons [3, 6, 7]. It remains unclear whether these improved outcomes in optimally debulked patients are due to the surgery or patient and disease factors [8].
Table 10.2
Definitions of cytoreduction
Complete cytoreduction | No visible residual disease |
Optimal | All residual deposits <1 cm maximal dimension |
Suboptimal | Residual deposits 1 cm or greater |
Cytoreductive Surgery
The aims of cytoreductive surgery is to (1) remove all sites of macroscopic disease, (2) assess areas for microscopic disease spread to allow accurate staging to guide treatment and inform prognosis and (3) minimise morbidity.
Evaluation of Patients for Surgery
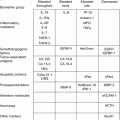
The patient should, if possible, be reviewed by a surgical and medical oncologist and then discussed at a multidisciplinary meeting with relevant imaging and blood tests (Table 10.3).
Table 10.3
Routine preoperative tests for patients with advanced ovarian cancer
Type of test | Test |
|---|---|
Routine blood tests | Full blood count, renal function, liver function, (including albumin), clotting profile, blood grouping |
Tumour markers | CA-125 (±CEA, CA19-9, HE-4, beta HCG, LDH, alpha fetoprotein, inhibin) |
Imaging | CT thorax, abdomen and pelvis or CT thorax and abdomen and MRI pelvis |
Cardiorespiratory | Electrocardiogram, ± echocardiogram, ± lung function, ± sleep studies, ± CPEX testing |
Other ± | Colonoscopy, gastroscopy, stoma counselling |
Initial management options include:
1.
Primary cytoreductive surgery
2.
Laparoscopy ± proceed to primary cytoreductive surgery
3.
Image-guided biopsy to establish ovarian cancer diagnosis and proceed to primary cytoreductive surgery
4.
Image-guided biopsy to establish ovarian cancer diagnosis and proceed to primary chemotherapy
5.
Palliative surgery
6.
Supportive care ± palliative chemotherapy
The use of image-guided biopsy prior to surgery (option 3 above) is reserved for cases in which the primary cancer is in doubt. Gastric cancer with omental disease, ascites and Krukenberg tumours may masquerade as an ovarian cancer and should be excluded if there is any doubt preoperatively [9].
Selection of Patients for Primary Cytoreductive Surgery
Survival benefit from primary cytoreductive surgery in advanced ovarian cancer is gained for patients where complete or optimal cytoreduction can be achieved rather than different prognoses according to specific sites of disease [10]. Furthermore, women with tumours that cannot be reduced to less than 2 cm will suffer surgical morbidity but probably derive no survival benefit from surgery [11]. A variety of predictive factors have been assessed. Bristow et al. identified a Gynecologic Oncology Group (GOG) performance status of ≥2, CT features of peritoneal thickening, peritoneal implants (≥2 cm), bowel mesentery involvement (≥2 cm), suprarenal para-aortic lymph nodes (≥1 cm), omental extension (spleen, stomach or lesser sac) and pelvic sidewall involvement and/or hydroureter to be most strongly associated with surgical outcome [12]. Using the scoring system (Table 10.4) in all patients with a score of less than four optimal or complete debulking was achieved, whereas in those with a score of 4 or greater, 87.5 % of patients were suboptimally debulked (Fig. 10.1) [12].
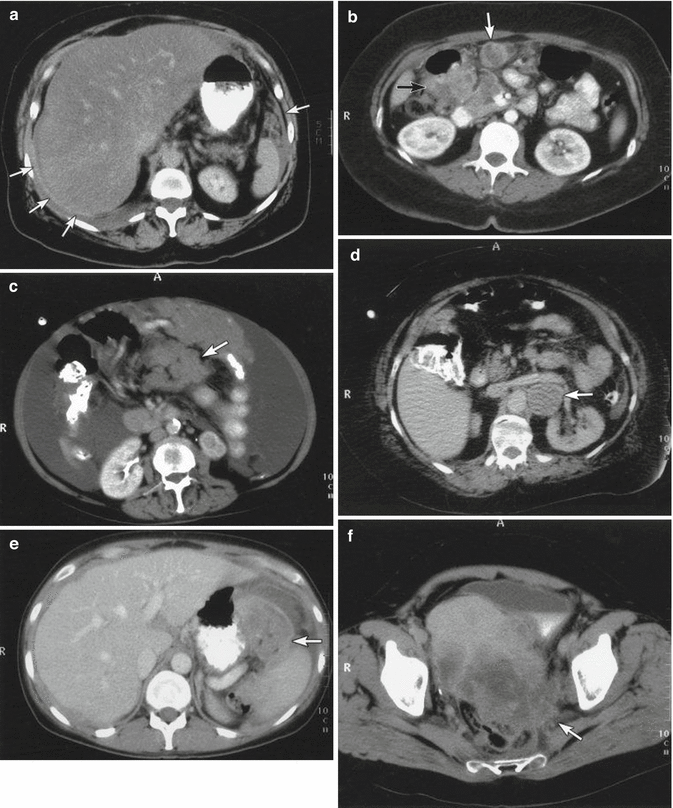
Table 10.4
Final model of predictive index parameters, including 13 radiographic features and GOG performance status >2
Predictive index parameter | Point value | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|
Peritoneal Thickening | 2 | 71.4 % (15/21) | 90.0 % (18/20) | 88.2 % (15/17) | 75.0 % (18/24) | 80.5 % (33/41) |
Peritoneal Implants ≥2 cm | 2 | 57.1 % (12/21) | 95.0 % (19/20) | 92.3 % (12/13) | 67.9 % (19/28) | 75.6 % (31/41) |
Small bowel mesentery disease ≥2 cm | 2 | 33.3 % (7/21) | 100 % (20/20) | 100 % (7/7) | 58.8 % (20/34) | 65.9 % (27/41) |
Large bowel mesentery disease ≥2 cm | 2 | 38.1 % (8/21) | 90.0 % (18/20) | 80.0 % (8/10) | 58.1 % (18/31) | 63.4 % (26/41) |
Omentum extension to stomach, spleen, or lesser sac | 2 | 42.9 % (9/21) | 85.0 % (17/20) | 75.0 % (9/12) | 58.6 % (17/29) | 63.4 % (26/41) |
Extension to pelvic sidewall, parametria, or hydroureter | 2 | 42.9 % (9/21) | 85.0 % (17/20) | 75.0 % (9/12) | 58.6 % (17/29) | 63.4 % (26/41) |
Ascites-large volume (seen on all cuts) | 2 | 38.1 % (8/21) | 90.0 % (18/20) | 80.0 % (8/10) | 58.1 % (18/31) | 63.4 % (26/41) |
Performance Status ≥2 | 2 | 33.0 % (7/21) | 95.0 % (19/20) | 87.5 % (7/8) | 57.6 % (19/33) | 63.4 % (26/41) |
Suprarenal paraaortic lymph nodes ≥1 cm | 2 | 23.8 % (5/21) | 100 % (20/20) | 100 % (5/5) | 55.6 % (20/36) | 61.0 % (25/41) |
Diaphragm or lung base disease ≥2 cm, or confluent plaque | 1 | 42.9 % (9/21) | 75.0 % (15/20) | 64.3 % (9/14) | 55.6 % (15/27) | 58.5 % (24/41) |
Inguinal canal disease or lymph nodes ≥2 cm | 1 | 19.0 % (4/21) | 100 % (20/20) | 100 % (4/4) | 54.1 % (20/37) | 58.5 % (24/41) |
Liver lesion ≥2 cm on surface, or parenchymal lesion any size | 1 | 14.3 % (3/21) | 90.0 % (18/20) | 80.0 % (8/10) | 58.1 % (18/31) | 56.1 % (23/41) |
Porta hepatis or gallbladder fossa disease ≥1 cm | 1 | 14.3 % (3/21) | 100 % (20/20) | 100 % (3/3) | 52.6% (20/38) | 56.1 % (23/41) |
Infrarenal paraaortic lymph nodes ≥2 cm | 1 | 14.3 % (3/21) | 100 % (20/20) | 100 % (3/3) | 52.6 % (20/38) | 56.1 % (23/41) |

Fig. 10.1
shows representative CT findings: (a) peritoneal thickening, (b) peritoneal implant ≥2 cm and large bowel mesentery disease ≥2 cm, (c) small bowel mesentery disease ≥2 cm, (d) suprarenal para-aortic lymph node ≥1 cm (the left renal vein can be seen running ventral to the tumour mass), (e) omental tumour extension to spleen and (f) tumour infiltration of parametrial soft tissue and extension to pelvic sidewall Bristow et al. [12]
Ferrandina et al. validated this approach in a European centre and identified five independent variables: ECOG performance status ≥2, CT findings of peritoneal thickening, peritoneal implants ≥2 cm, bowel mesentery involvement, suprarenal aortic lymph nodes ≥1 cm and diaphragmatic disease (widespread infiltrating carcinomatosis or confluent nodules) [13]. With each of these allocated a score of 1, a predictive index value can be calculated (PIV) with corresponding rates of unnecessary surgery and also inappropriate unexploration.
Vergote and colleagues report the Leuven criteria to determine patients where primary cytoreductive surgery should not be performed [4].
There is currently no perfect model that can completely predict patients where optimal debulking may be achieved so that patients do not receive unnecessary surgery. Variation between surgeons and centres makes any predictive model difficult to apply universally and the lack of reproducibility of CT interpretation highlights the caution that should be observed with any model [14]. In particular radical upper abdominal procedures have been introduced to many gynaecological oncology centres with associated improvements in optimal debulking and survival and will alter rates of optimal and complete debulking [15]. Chi et al. reported a significant increase in both cytoreduction, survival and progression-free survival when extensive upper abdominal procedures were performed on patients with advanced ovarian cancer [15] (Fig. 10.2).
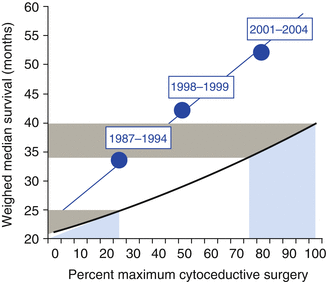

Fig. 10.2
Median overall survival as a function of percent maximum or optimal cytoreductive surgery. Memorial Sloan Kettering Cancer Center survival 1987–2004 superimposed on model by Bristow et al. (Modified with permission Bristow et al. [3])
This often requires close working with hepatobiliary and colorectal surgeons both for preoperative evaluation and intraoperative support. These procedures are associated with significant surgical morbidity and it is essential to audit quality of life alongside disease specific measures as this approach becomes more widespread.
For many patients although there may be no survival benefit from primary surgery if optimal debulking is not achieved, surgery may improve symptoms such as those from large ovarian cysts or obstructed bowel and establish diagnosis.
Operative and Perioperative Management
Preoperative evaluation will help determine the likely extent of surgery in particular the likelihood of bowel resection, stoma formation and upper abdominal procedures. It is important that any relevant multi-professional support is available if procedures outside the repertoire of the lead surgeon may be performed. For patients where a stoma is likely, preoperative stoma siting and counselling are useful not only to ensure a good anatomical location of any stoma but also help with psychological aspects of bowel diversion. Patients should also be counselled about the potential benefits and risks of intraperitoneal chemotherapy if optimal or complete debulking is achieved and consent taken for intraperitoneal catheter placement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree