Introduction
“Insulin neuritis” is a historical term for acute painful neuropathy related to the initiation of insulin treatment and the subsequent improvement in glycemic control in patients with diabetes. Caravati was the first to describe this condition in 1933 in a type 1 diabetic patient who developed severe pain following rapid glycemic control with insulin . Since the initial report in 1933, several small case series described an association between acute onset of painful neuropathy and a rapid correction of hyperglycemia . The term “insulin neuritis” does not correctly describe the condition since this form of painful neuropathy can occur after any antidiabetic agent is administered. Thus it has been proposed that treatment-induced neuropathy of diabetes (TIND) would be a more descriptive name for it . The main clinical manifestations are severe treatment-resistant pain and autonomic dysfunction secondary to a rapid decrease in hemoglobin A 1c , in some cases accompanied by worsening diabetic retinopathy (DR) and nephropathy.
Gibbons and Freeman retrospectively reviewed all individuals referred to a tertiary care diabetes neuropathy clinic over 5 years . They identified a subset of patients with type 1 diabetes and painful neuropathy, preceded by a significant improvement in HbA 1c . Accordingly, they proposed the following three clinical criteria for the diagnosis of TIND: (1) a decrease in HbA 1c ≥2% (22 mmol/mol) over 3 months; (2) the acute onset of neuropathic pain and/or autonomic dysfunction, developing acutely and severely enough to cause the patient to seek medical attention; and (3) the neuropathic pain or autonomic dysfunction beginning within 8 weeks of the decrease in HbA 1c . In addition, they noticed that patients who developed TIND typically had very high pretreatment HbA 1c >10% (86 mmol/mol). In this cohort, none of the patients had newly diagnosed type 1 diabetes. Nevertheless, there are several case reports of patients with a recent diagnosis of type 1 diabetes and TIND, even in children and adolescent patients . Data regarding the prevalence of TIND in type 2 diabetes are even more limited, but TIND appears more frequently reported in patients with type 1 diabetes. This chapter will review the data available on the epidemiology of TIND, as well as its mechanisms, risk factors, clinical presentation, diagnostic methods, and management.
Potential mechanisms and pathogenesis
Although several theories have been proposed, the pathophysiology of TIND is not fully understood. In diabetic polyneuropathy (DPN), the most typical finding is a mixed fiber neuropathy with both myelinated and unmyelinated fibers affected. However, the typical clinical presentation of TIND is a length-dependent, symmetrical small-fiber neuropathy (SFN) associated with sensory and/or autonomic symptoms. In SFN, thinly myelinated Aδ-fibers and unmyelinated C-fibers are affected . Large fibers are not affected, and thus nerve conduction studies (NCSs) remain normal. However, some TIND patients have shown mild sensory, axonal neuropathy in NCSs .
One suggested pathogenetic mechanism of TIND is the formation of arteriovenous shunting and endoneurial ischemia as a result of a steal effect from the proliferating epineurial vessels . The coincident changes in the retina and kidney support this microvascular mechanism.
Another proposed mechanism is relative hypoglycemia, since the previous long-standing hyperglycemia may have led to energy-dependent failure of axonal transport . Consequently, a rapid reduction in the blood glucose level may result in energy deficiency. Unmyelinated fibers with a large surface area-to-size ratio, compared to myelinated fibers, could be particularly susceptible to a sudden reduction in glucose supply. In a cell model of diabetic neuropathy, relative glucose deprivation resulted in cellular apoptosis . In addition, pro-inflammatory cytokines have been proposed to have a role in the development of hyperalgesia and autonomic dysfunction . Moreover, fiber regeneration and spontaneous firing of regenerating nerve fibers may play a role in the pain mechanisms .
One important predisposing factor for chronic neuropathic pain in diabetes patients is an impaired function of the descending, inhibiting pain pathway of the dorsal horn of the spinal cord, which has been shown, for example, in a brain imaging study that compared patients with painful and nonpainful DPN , and in a duloxetine intervention study of painful diabetic neuropathy . However, the function of the descending, inhibiting pain pathway has not been studied in TIND patients thus far.
Epidemiology
The prevalence of TIND in the general population is unknown. The available data are based on case reports and small, nonpopulation-based studies . The largest cohort thus far was studied by Gibbons and Freeman . Over 5 years, 954 patients with type 1 diabetes were retrospectively evaluated for diabetic neuropathy at a tertiary center for neurologic disorders, and 10.9% of these individuals met the suggested criteria for TIND. The study design did not allow assessment of the population-based prevalence of the disorder. However, the findings indicated that TIND might be more common than previously believed . There is also no precise information on the incidence of TIND.
Risk factors
The factors that predispose patients to TIND are unknown. Gibbons and Freeman reported an association between the severity of TIND and the extent of the change in HbA 1c ; the risk of TIND depended on the reduction in HbA 1c level. TIND had a 20% chance of occurring when HbA 1c levels decreased during 3 months by two to three units and more than an 80% chance when HbA 1c levels decreased by more than four units . Young women with type 1 diabetes and a history of eating disorders appear to be at increased risk of developing TIND . Moreover, TIND has been described in the context of rapid improvements in glycemic control and weight loss, both after bariatric surgery and as a result of a low-calorie diet. Common denominators seem to be weight loss, parallel with the rapid correction of glycemic control and nutritional deficiencies. Painful neuropathy and autonomic dysfunction similar to TIND have been reported in some patients after bariatric surgery, attributed to malnutrition and deficiencies in micronutrients such as vitamin B12 .
Notably, there are no published neuropathological or neurophysiological data regarding patients with TIND prior to the onset of neuropathic symptoms, nor are there prospective studies that assessed the correlation between the development of TIND and the change in HbA 1c level or other biomarkers. TIND has not been as well described in newly diagnosed patients with type 1 diabetes, although case reports do exist . Notably, adult-onset type 1 diabetes is usually a slowly progressive disease, where symptoms and weight loss can go unnoticed for months . This period of undiagnosed and untreated hyperglycemia often results in high HbA 1c at diagnosis and, frequently, in ketoacidosis. Thus neural dysfunction may already be present when type 1 diabetes is diagnosed.
Clinical presentation
The typical clinical presentation of TIND is an acute or subacute onset of severe neuropathic pain and autonomic dysfunction within 2–8 weeks of the improvement in glucose control ( Table 12.1 ). Typically, patients experience a feeling of constant burning or lancinating pain in the feet. In addition, patients have reported tingling, numbness, chills, a feeling of tightness or pressure, and sometimes itching. Patients frequently experience pain induced by touch (allodynia) where, for example, skin contact with sheets or socks feels painful, or thermal dysesthesia and allodynia, where a hot shower may be perceived as unpleasant or painful .
|
Moreover, weakness, painful muscle cramps in the legs, and restless legs syndrome have been described as TIND symptoms. Usually, such symptoms begin in the distal parts of the lower limbs and slowly progress proximally in a length-dependent fashion. In some cases, the symptoms spread to the upper limbs or present in patches in a nonlength-dependent pattern. They typically get worse when the person is at rest and may interfere with sleep.
In addition to neuropathic pain, a patient may present with various symptoms of autonomic dysfunction, which are probably underreported or dismissed due to severe neuropathic pain. Autonomic dysfunction may be completely asymptomatic and detected only by thorough clinical examination. Symptoms of dysautonomia are more likely to be identified through detailed questioning or by using validated questionnaires . Occasionally, autonomic symptoms may be the presenting feature. Severe autonomic failure may also be seen in some cases. There appears to be a relationship between the severity of autonomic failure and the decrease in HbA 1c . Common symptoms of cardiovascular autonomic dysfunction are arrhythmias or palpitations (resting tachycardia), orthostatic intolerance, or orthostatic hypotension with syncope. Gastrointestinal autonomic dysfunction may manifest with early satiety, nausea, vomiting, or dyspepsia due to oesophagic dysmotility or gastroparesis or with constipation, diarrhea, or fecal incontinence. Possible genitourinary disturbances are erectile dysfunction and bladder dysfunction. Moreover, hypoglycemia unawareness and sudomotor dysfunction with either increased or decreased sweating may be seen.
Diagnostic methods
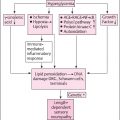
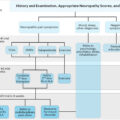
In general, the diagnosis of typical diabetic peripheral neuropathy is clinical . DPN usually involves a mix of large-fiber neuropathy and SFN, and of the patients with diabetic neuropathy, 25% report painful neuropathy . TIND is considered primarily an SFN, thus its diagnosis may be challenging. Notably, electromyoneurography (EMNG) remains normal in SFN, and other tests are needed to confirm suspected SFN. However, DPN may be preexisting in TIND patients with a long history of diabetes and inadequate glycemic control. NCS (EMNG) is the gold standard as a confirmatory test in painful DPN diagnostic work-up.
The diagnosis of TIND is based on the patient’s history and symptoms, as well as a clinical examination that includes a bedside assessment of their sensory and motor activities ( Table 12.2 ). At the bedside, small-fiber function is examined by testing the patient’s responses to different stimuli, including heat, cold, pinprick, pressure, and touch (with a metal roller or object, sharp wooden cocktail stick, finger compression, painter’s brush, and a cotton tuft, respectively). Pinprick and temperature sensations are mediated via small nerve fibers, whereas large nerve fibers mediate monofilament sensation, vibration sensation, and proprioception. For motor testing, heel-toe walking and reflexes are important. Sensory responses may include increased painful sensation (hyperalgesia), a nonpainful stimulus (e.g., heat, cold, painter’s brush, or light touch) causing pain (allodynia), a stimulus causing an unpleasant sensation (dysesthesia), or a stimulus causing a diminished sensation (hypoesthesia).
| Clinical examination |
|
| Nerve conduction studies (EMNG) |
|
| Small-fiber testing |
|
| Autonomic testing |
|
| Laboratory tests |
|
| Differential diagnosis |
|
The tests that can confirm TIND-related polyneuropathy and/or SFN are EMNG, skin biopsy ( Fig. 12.1 ), quantitative sensory testing (QST), autonomic function tests according to symptoms, and exclusion of other causes ( Table 12.2 ).
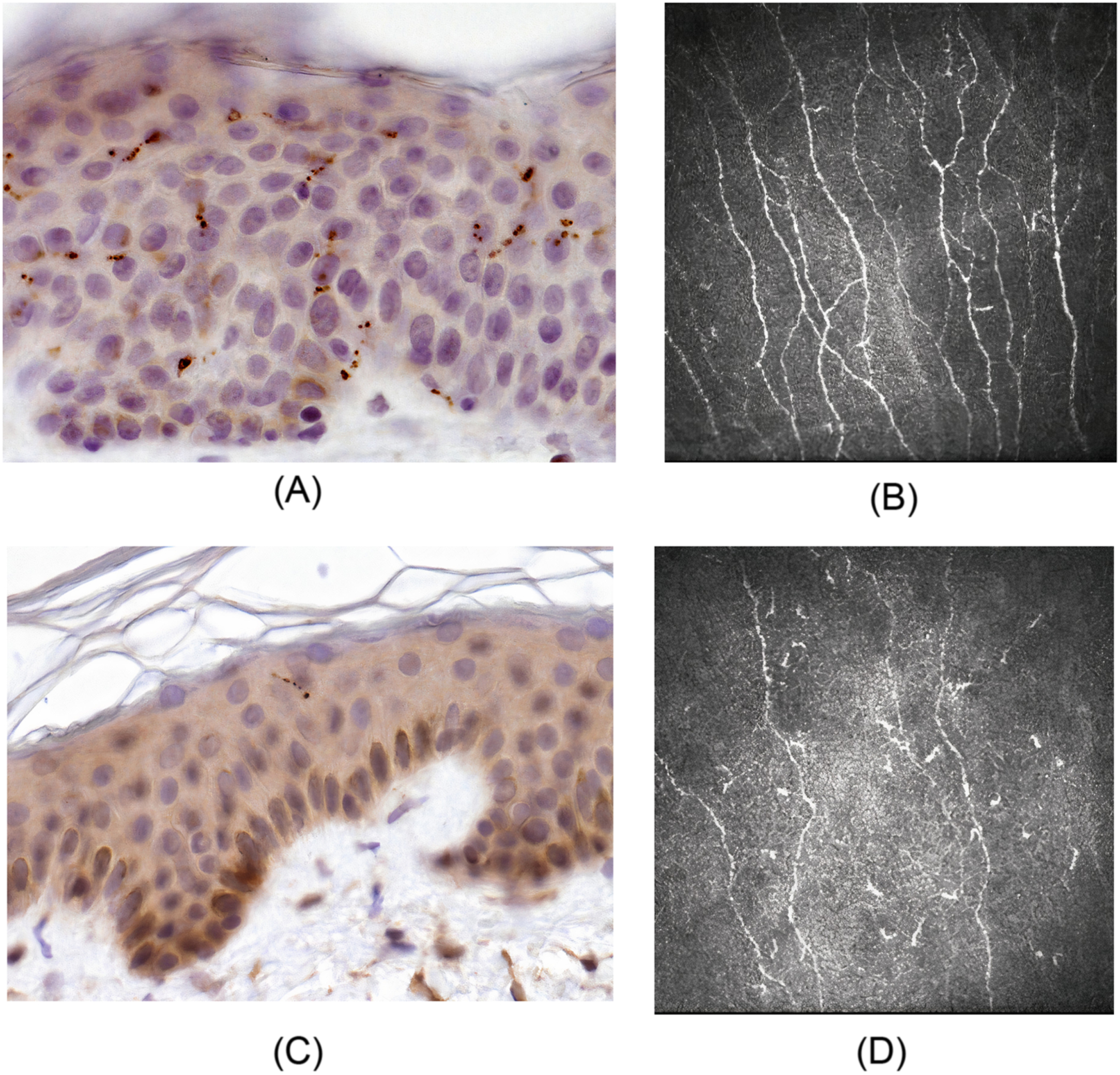
Skin punch biopsy and the assessment of intraepidermal nerve fiber density (IENFD) is the most sensitive tool and the gold standard for diagnosing SFN . However, a skin biopsy is invasive and thus primarily used in specialized clinics. In clinical practice, skin biopsy should most likely be used more often in the SFN diagnostic procedure. Fig. 12.1 demonstrates skin biopsies from a healthy control and a patient with severe TIND.
QST is a psychophysical measurement that requires relevant patient cooperation in evaluating responses to stimuli. In recent years, a more extensive QST has been developed to cover, not only thermal thresholds, but all the sensory modalities concerned for SFN. This larger QST is called DFNS (Deutscher Forschungsverbund Neuropatischer Schmertz)-QST, according to the German Research Network on Neuropathic Pain, and it is currently in clinical and research use in several centers . With DFNS-QST, a patient-specific sensory profile can be developed . This may be a helpful tool for improving the accuracy of SFN diagnostics in the future.
Corneal confocal microscopy (CCM) is a promising repeatable, noninvasive method for assessing small-fiber damage in diabetic peripheral neuropathy . In several studies, measures of CCM have correlated with IENFD, the severity of painful neuropathic symptoms and quality of life, and autonomic neuropathy measures in patients with painful DPN . However, a study attempting to detect DPN from a cohort of confirmed and unconfirmed DPN, and healthy controls by assessing CCM remained negative, although corneal nerve fiber density was lower in patients with DM compared with controls . Fig. 12.1 demonstrates corneal confocal microscopy and nerve fiber density in a healthy control and a patient with severe TIND. Further studies are needed to validate CCM for routine clinical use and in the assessment of TIND.
Methods that assess sweat gland density and distribution, and stimulated sweat production, have not yet been validated for routine clinical use. Other confirmatory tests of SFN include laser Doppler flare imaging studies and positron emission tomography scans. These have no exact role in routine clinical diagnosis.
If a patient under suspicion of having TIND shows atypical features, such as asymmetric presentation of neuropathy or motor predominance, a neurological consult, as in other forms of diabetic neuropathy, should be considered. Additional testing options include measuring the patient’s serum vitamin B12 levels, conducting thyroid function tests, serum protein electrophoresis with immunofixation, and assessing for markers of autoimmune disorders.
Management of symptoms associated with treatment-induced neuropathy of diabetes
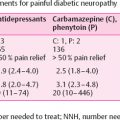
Typically, patients with TIND experience severe, subacute neuropathic pain, and over-the-counter analgesics usually do not alleviate it. In pain management, the same medications are used as in other forms of painful diabetic neuropathy [tricyclic antidepressants, gabapentinoids, and serotonin-norepinephrine reuptake inhibitors (SNRIs)]. However, due to its rapid start, the neuropathic pain may be more severe than usual in painful diabetic neuropathy, and the pain medication may need rapid escalation and/or the addition of several neuropathic pain medications. Correspondingly, the intake of this medication should be gradually reduced as the pain dissipates.
Treatment of neuropathic pain may not alter the natural course of TIND but is essential to improving the patient’s quality of life and pain control. In some cases of TIND, maximizing treatment with a single neuropathic pain agent may help. Although tricyclic antidepressant medications can be used, the anticholinergic side effects may be worsen autonomic dysfunction. In those cases, gabapentinoids or SNRIs may be a good alternative. Eventually, neuropathic pain may be resolved with stable glycemic control. It is essential to inform the patient about the disease course and its spontaneous tendency to heal, although healing may take anywhere from 6 to 24 months. Particularly in patients with newly diagnosed type 1 diabetes, the burden of a recent diagnosis combined with severe neuropathic pain may be challenging. Chronic pain is also associated with worse self-management of diabetes and higher blood glucose levels . Thus the patient may need psychological support. Historically, it was believed that the pain related to TIND could be relieved through “permissive hyperglycemia.” However, no data support that view. On the contrary, it appears that allowing hyperglycemia increases the risk of DPN and new episodes of TIND , thus sustained glycemic control is recommended. Pharmacologic treatment of autonomic dysfunction is challenging, although symptomatic treatment of tachycardia, gastrointestinal symptoms, and erectile dysfunction may improve a patient’s quality of life. Diabetic autonomic neuropathy is considered an irreversible, progressive form of neuropathy. Importantly, autonomic dysfunction occurring with TIND is usually reversible and resolves along with the other symptoms.
Outcomes of treatment-induced neuropathy of diabetes and association with other microvascular complications
Data on long-term follow-up of patients with TIND are limited. Based on previous case reports and small groups of studied cohorts, manifestations of TIND are typically resolved within 1–2 years. In a recent study that is, to our knowledge, the only follow-up study that has been conducted on this topic, 26 individuals with type 1 diabetes and TIND were followed for 8 years . The neuropathic pain scores improved within 18–36 months after the onset, which supports previous data. Interestingly, although the patients in both groups were similar at baseline, the outcomes diverged substantially, depending on the glycemic control during follow-up. For the 19 patients who maintained stable glycemic control during follow-up, their autonomic scores, NCSs, IENFDs, pain scores, and microvascular complications were observed to have improved.
In contrast, a subgroup with unstable glycemic control during follow-up experienced new TIND episodes, persistent neuropathic pain, and demonstrated deterioration in all measures. Notably, despite severe TIND at baseline, stable glycemic control allowed almost complete recovery in both structural and functional neural parameters. The neural damage in diabetic peripheral polyneuropathy is considered irreversible, as intervention studies have failed to reverse the process. As supported by this study, TIND is considered a reversible form of diabetic neuropathy, and, importantly, it has been observed in structural neural outcomes in patients with a long history of type 1 diabetes.
Several studies have reported the development or progression of DR and microalbuminuria in association with TIND; however, data are limited . In a follow-up study conducted by Gibbons and Freeman, more than half of those with TIND had no DR, and only 3% had proliferative retinopathy at baseline . After the development of TIND, 90% had either severe nonproliferative or proliferative retinopathy. The pathophysiology of this phenomenon is not well understood but supports a common microvascular etiology. Early worsening of DR is a well-described phenomenon associated with the rapid improvement in glycemic control in patients with both type 1 and type 2 diabetes . However, longitudinal studies have demonstrated that intensive glucose control is the cornerstone of the reduction of diabetic microvascular complications. Despite the initial worsening, intensively treated patients have demonstrated a sustained protective effect of improved glycemic control during follow-up. Therefore it is likely that the deterioration of DR seen in patients with TIND can be attributed to improved glycemic control and not neuropathy.
In a follow-up study of patients with TIND, the patients with inadequate glycemic control after experiencing TIND had a substantial decline in renal function, and some patients progressed to hemodialysis .
Discussion
TIND is a rare, underreported, and often unidentified form of diabetic neuropathy. Its prevalence in the general population, though unknown, appears to be increasing . During the last decades, diabetes guidelines have emphasized the importance of achieving treatment goals to prevent microvascular complications. Thus increased attention to reaching adequate glycemic control could be one explanation for the suggested increase in TIND prevalence. Moreover, emerging potent antidiabetic agents and new diabetes technology, including flash glucose monitoring, continuous glucose monitoring, and hybrid closed-loop systems, allow better glycemic control.
Nonetheless, there is a mismatch between the number of patients whose glycemic control rapidly improves and those who develop TIND. It is also not fully understood why only some patients with neuropathy experience painful symptoms. Patients who develop TIND may also have an unidentified predisposing condition. The association between variants in the voltage-gated sodium channel Na V 1.7 encoded by the SCN9A gene and TIND has not been studied.
No recommendations have been made to identify individuals at risk or prevent the development of TIND. Based on the available data, a precaution in the intensification of treatment is essential in long-standing hyperglycemia, especially in young females who have experienced weight loss . In a newly diagnosed type 1 diabetic patient, hyperglycemia may have lasted for months; thus symptoms lasting for weeks or months, high HbA 1c , or a history of ketoacidosis may indicate an increased risk for TIND. Therefore TIND could be even more common in patients with new-onset type 1 diabetes, as initiation of insulin treatment almost invariably results in rapid improvement of glycemic control. Gibbons et al. propose limiting the reduction in HbA 1c to less than 2% (22 mmol/mol) over 3 months , although prospective data on prevention are lacking. Thus, based on the available data, stable glycemic control is recommended in the management of individuals with type 1 diabetes and TIND. No longitudinal follow-up studies of people with type 2 diabetes and TIND have been conducted. Early worsening of DR is a known phenomenon associated with rapid improvement in glycemic control. Accordingly, eye status should be assessed in patients at increased ocular risk before antidiabetic treatment is intensified. Moreover, screening for both retinopathy and microalbuminuria could be recommended in all TIND presentations, irrespective of diabetes duration.
There are several pharmacological treatment options for painful diabetic neuropathy; however, disease-modifying therapies for TIND, and even diabetic neuropathy in general, are still lacking. Importantly, painful neuropathy is both underreported and inadequately treated . Severe TIND often has debilitating consequences for the patient, including interference with daily activities, work disability, and reduced health-related quality of life. For patients at risk of developing TIND, regularly conducted questioning with a pain scale and patient pain drawings could allow for early recognition and pain management. Whether patients with TIND are at risk of later diabetic neuropathy is not known. However, based on the available data, it appears that, at least in patients with type 1 diabetes, stable glycemic control prevents the progression of neuropathy and even improves neural markers after a 9-year follow-up . Regarding diabetic peripheral neuropathy, the American Diabetes Association recommends assessing for it in patients who have had type 1 diabetes for 5 years, and at diagnosis in patients with type 2 diabetes or those with impaired glucose tolerance or neuropathic symptoms . Importantly, TIND should be considered in any diabetes patient experiencing acute neuropathic pain or exhibiting signs of autonomic dysfunction regardless of the type or duration of diabetes or the current treatment.
Many questions remain unanswered. The optimal rate for reestablishing glycemic control that prevents TIND but does not compromise the future risk of microvascular complications is unknown. Thus far, treatment for TIND comprises pain management, symptomatic treatment of autonomic dysfunction, and optimization of glycemic control. Further research into the pathophysiology, risk factors, treatment, and prevention of TIND is needed.
Declaration of competing interests
None
Acknowledgments
The authors want to thank Prof. Anders Paetau for the skin biopsy images.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree