Author
Study design
Setting
Population
Treatment line
Patients, n
Patients with NCCRCC, n
NCCRCC histologies
Targeted agent
Ratain et al. [5]
2
Metastatic
RCC
2nd
152
15
PRCC
Sorafenib
Stadler et al. [6]
EAT
Metastatic
RCC
≥2nd
2504
202
Mixed
Sorafenib
Beck et al. [7]
EAT
Metastatic
RCC
≥2nd
1159
118
Mixed (PRCC)
Sorafenib
Plantade et al. [8]
2
Metastatic
RCC
≥2nd
28
PRCC
Sorafenib
Gore et al. [10]
EAT
Metastatic
RCC
≥2nd
4371
588
Mixed
Sunitinib
Choueiri et al. [11]
2
Metastatic
RCC
≥2nd
53
41 papillary and 12 chromophobe
Sorafenib or sunitinib
Molina et al. [12]
2
Metastatic
RCC
≥2nd
23
Mixed 8 with PRCC
Sunitinib
Lee et al. [13]
2
Metastatic
RCC
≥2nd
31
Mixed (PRCC, ChRCC, Xp11.2 translocation)
Sunitinib
Hudes et al. [14]
3
Metastatic
RCC
1st
526
20 %
Mixed
Temsirolimus
Dutcher et al. [16]
3
Metastatic
RCC
1st
209
37
Mixed
Temsirolimus
Blank et al. [18]
EAT
Metastatic
RCC
≥2nd
1367
75
Mixed
Everolimus
Choueiri et al. [22]
2
Metastatic
RCC
≥2nd
74
PRCC
Foretinib
Gordon et al. [25]
2
Metastatic
RCC
≥2nd
45
PRCC
Erlotinib
Oudard et al. [28]
2
Metastatic
RCC
≥2nd
23
CDRCC
Gemcitabine and platinum
Golshayan et al. [37]
Retrospective
Metastatic
Sarcomatoid
≥2nd
43
–
RCC with SD
Sunitinib, sorafenib, bevacizumab
Haas et al. [39]
2
Metastatic
Sarcomatoid
1st
39
–
RCC with SD
Doxorubicin-gemcitabine
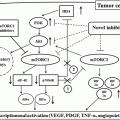
13.2 Targeted Therapies for Metastatic Papillary RCC (PRCC)
Compared with CCRCC, metastatic PRCC is rare, but once the disease is systemic, the prognosis is just as bad or worse. Immuno(chemo)therapy has been inactive in papillary RCC [3], and therefore, until recently, there was no rational therapeutic option for this tumor subtype. PRCC has been further divided into type 1 and type 2 subtypes; it can occur sporadically or as part of a hereditary syndrome. Comparative genomic microarray analyses showed two highly distinct molecular PRCC subclasses well associated with morphologic subclasses. The first class, with excellent survival, corresponded to three histologic subtypes: type 1, low-grade type 2, and mixed type 1/low-grade type 2 tumors. The second class, with poor survival, corresponded to high-grade type 2 tumors [4].
13.2.1 The Efficacy of Sorafenib to PRCC
Ratain et al. [5] firstly reported the efficacy of sorafenib in patients with metastatic PRCC in 2006. In their phase II clinical trial, they used sorafenib to treat 152 and 15 patients with metastatic CCRCC and PRCC, respectively. The antitumor effect in PRCC was similar to that of the CCRCC population. Two patients with PRCC showed partial response (PR), and an additional three had tumor shrinkage of 25–49 %, suggesting that sorafenib could have significant efficacy in patients with metastatic PRCC.
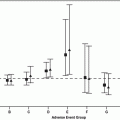
The 202 patients with NCCRCC enrolled in the US Advanced RCC Sorafenib (US-ARCCS) expanded access trial (EAT) included 107 patients with PRCC [6]. The rate of clinical benefit (complete response [CR] + PR + stable disease [SD] for at least 8 weeks) was 84 % in patients with PRCC similar to that in the entire population. The median progression-free survival (PFS) in the overall population was 24 weeks (95 % CI: 22–25 weeks) and was the same when patients with NCCRCC were excluded. The median PFS in patients with NCCRCC was 21 weeks; the median overall survival (OS) in this cohort was 40 weeks compared with 50 weeks (95 % CI: 46–52 weeks) in the overall population. Reported adverse events (AEs) did not differ from those observed in patients with CCRCC. The authors concluded that sorafenib was well tolerated and had significant antitumor activity in patients with metastatic PRCC. Beck et al. [7] presented similar results from the European (EU)-ARCCS trial, an open-label, non-comparative phase III study. That study included 15 PRCC histologies. Of these patients, one with PRCC achieved a PR with sorafenib (objective response rate [ORR] 3.4 %), compared with an ORR of 9.3 % in patients with CCRCC. Two patients with papillary histology exhibited tumor shrinkage.
On the contrary, within a recent French study [8], there were no objective responses in 28 patients with advanced PRCC treated with sorafenib. However, the median PFS and overall survival was 5.7 and 19.6 months, respectively.
13.2.2 The Efficacy of Sunitinib to PRCC
Within the US-sunitinib EAT, 276 evaluable patients with NCCRCC received sunitinib (4 weeks on, 2 weeks off), in most of whom previous cytokine-based therapy had failed [9]. Even though the results were less notable than for CCRCC, sunitinib also showed significant activity against NCCRCC. Overall response and disease control rates for NCCRCC compared with all RCC subtypes were 5.4 % vs 9.3 % and 47.0 % vs 52.4 %, respectively. Unfortunately, the authors did not discriminate between different NCCRCC subtypes. In 2009, Gore et al. updated the results of the US-sunitinib EAT including 588/4371 patients with NCCRCC, comprising 13 % of the overall study population [10]. In this study, the overall median PFS was 10.9 months (95 % CI: 10.3–11.2 months), and the median OS was 18.4 months (95 % CI: 17.4–19.2 months); the corresponding survival times in the subgroup of patients with NCCRCC were 7.8 months (95 % CI: 6.8–8.3 months) and 13.4 months (95 % CI: 10.7–14.9 months), respectively [10]. Although the sunitinib benefit in NCC histologies appeared lower than in the overall population, the median OS compares favorably with historical data [3].
Sunitinib efficacy was also observed in a retrospective analysis of 53 patients with PRCC treated with sunitinib (n = 20) or sorafenib (n = 33) at five cancer centers in France and the USA [11]. Among the sunitinib-treated patients, 2 of 13 patients with PRCC achieved a PR (15 %), and median PFS in this histological subtype was 11.9 months. None of the 28 patients with PRCC treated with sorafenib achieved an objective response. The median PFS in this cohort was 5.1 months, significantly less than the 11.9 months achieved by sunitinib treatment in patients with PRCC (P < 0.001).
Data from several prospective phase II studies of sunitinib in advanced NCCRCC have been presented or published. For the most part, there was a low response rate to sunitinib (ORR 0–7 %), although the majority of patients showed SD [12]. However, a recently published phase II study of 31 patients with NCCRCC (papillary, n = 22; chromophobe, n = 3; unclassified, n = 5; and Xp11.2 translocation, n = 1) reported an overall ORR of 36 % (95 % CI: 19 % to 52 %) and median PFS of 6.4 months (95 % CI: 4.2–8.6 months). The median OS had not been reached, but the 1-year survival rate was 65 % [13].
13.2.3 The Efficacy of mTOR Inhibitors to PRCC (NCCRCC)
In 2007, a phase III trial compared the efficacy and safety of temsirolimus along with temsirolimus in combination with interferon α (IFNα) or IFNα alone for the first-line treatment for poor-risk RCC [14]. This phase III study is of particular interest when considering the treatment of NCCRCC, as it is the only phase III RCC trial to date with NCC histology representation; of the 626 patients enrolled, 20 % had RCC of NCC histology. Dutcher et al. [15, 16] presented a subgroup analysis of the temsirolimus vs IFNα trial, comparing the activity of temsirolimus and IFNα in metastatic NCCRCC and the efficacy of temsirolimus in CCRCC and NCCRCC. That study included only treatment-naive patients with an intermediate and poor risk, and 76 % of those with NCCRCC had PRCC. In this population, the median OS was 11.6 months with temsirolimus and 4.3 months with IFNα (HR 0.49; 95 % CI: 0.29–0.85 months); median PFS, based on independent assessment, was 7.0 months with temsirolimus and 1.8 months with IFNα (HR 0.38; 95 % CI: 0.23–0.62 months). These outcomes are at least comparable with those for patients with clear cell RCC. The impact of temsirolimus on health-related quality of life also showed a trend for superiority over IFNα in RCC of NCC histology [17]. Taken together, these analyses strongly suggest that temsirolimus provides clinical benefit for the first-line treatment of NCCRCC.
Data on the use of the mTOR inhibitor everolimus in NCCRCC are limited, although a subgroup analysis of patients with NCCRCC enrolled in the RAD1001 Expanded Access Clinical Trial in RCC (REACT) was presented at the ASCO 2012 Genitourinary Cancers Symposium [18]. REACT enrolled RCC patients of any histology who were intolerant to, or had progressed on, VEGFR inhibitors; of 1367 patients enrolled, 75 patients (5.5 %) had NCCRCC. Median treatment duration was similar in the NCC subgroup and in the overall REACT population (12.14 weeks versus 14.0 weeks, respectively), as was the ORR (1.3 % versus 1.7 %) and rate of stable disease (49.3 % versus 51.6 %), suggesting that everolimus shows similar results in NCCRCC compared with CCRCC.
13.2.4 Other Targeted Therapies in PRCC
One causative gene responsible for hereditary papillary RCC has been identified on chromosome 7q31 and encodes the receptor tyrosine kinase MET proto-oncogene [19]; this proto-oncogene is also dysregulated/duplicated in a significant proportion of sporadic type 1 cases [20], but the exact role of c-MET in the development of PRCC is yet unknown. Foretinib is an oral, multikinase inhibitor targeting VEGFR-2, MET, and other receptors. Preliminary data from a phase I trial of advanced PRCC [21] suggested that the agent may have activity in this setting. In a recently reported multicenter phase II study of patients with sporadic and hereditary PRCC (N = 74), foretinib was associated with an ORR of 13.5 % (while tumor shrinkage was reported in 50 out of 68 patients), a disease stabilization rate (ORR + stable disease) of 88 %, median PFS of 9.6 months, and 1-year OS of 70 % (median OS not reached) [22]. Toxic effects were manageable and typical of anti-VEGF therapy.
EGFR pathway has also been implicated in the development of metastatic RCC [23, 24]. Gordon et al. [25] evaluated the efficacy of erlotinib, an oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor in 45 patients with advanced PRCC. The overall RR was 11 % (five of 45 patients; 95 % CI, 3 % to 24 %), and the disease control rate was 64 % (i.e., five partial response and 24 stable disease). The median overall survival time was 27 months (95 % CI, 13 to 36 months). Probability of freedom from treatment failure at 6 months was 29 % (95 % CI, 17 % to 42 %). There was one grade 5 AE of pneumonitis, one grade 4 thrombosis, and nine other grade 3 AEs. The design of future trials of the EGFR axis in PCC should be based on preclinical or molecular data that define appropriate patient subgroups, new drug combinations, or potentially more active alternative schedules. Clinical phase II studies are ongoing to determine the efficacy of the combination of bevacizumab and erlotinib as a treatment for patients with metastatic NCCRCC (NCT01399918) or papillary RCC (NCT01130519) in the USA (Table 13.2). These studies might provide new insights on sequential or combinational therapy for NCCRCC such as PRCC.
Table 13.2
Summary of ongoing and planned trials in NCCRCC
Drug
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|